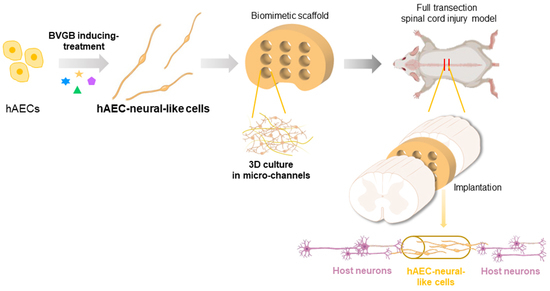
Therapeutic Effect of Biomimetic Scaffold Loaded with Human Amniotic Epithelial Cell-Derived Neural-like Cells for Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. hAECs Preparation
2.2. Establishment of BGVB Inducement Approach
2.3. GelMA Preparation and Biomimetic Spinal Cord Scaffold Printing
2.4. Flow Cytometry
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Quantitative Real-Time PCR
2.7. Pre-Labelling of hAECs
2.8. hAECs Seeding and hAEC-Neural-like Cells Induction on Biomimetic Spinal Cord Scaffold
2.9. Scanning Electron Microscopy and Imaging
2.10. Animals
2.11. T10 Full Transection Injury and Post-Surgical Care
2.12. EMG Recording and Cortical Stimulation
2.13. Behavioral Assessments
2.14. Immunofluorescence and Imaging
2.15. Quantification and Statistical Analysis
3. Results
3.1. The Basic Characteristics of Human Amniotic Epithelial Cells
3.2. The Establishment of BGVB Inducing-Treatment Approach
3.3. The Establishment of 3D Biomimetic Spinal Cord Scaffold Loaded with hAEC-Neural-like Cells
3.4. The Biomimetic Scaffold Loaded with hAEC-Neural-like Cells Replenished Neural Cells and Improved Locomotion in SCI Rat Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mcdonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A clinical practice guideline for the management of acute spinal cord injury: Introduction, rationale, and scope. Glob. Spine J. 2017, 7, 84S–94S. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Bannick, M.S.; Montjoy-Venning, W.C.; Lucchesi, L.R.; Dandona, L.; Dandona, R.; Hawley, C.; Hay, S.I.; Jakovljevic, M.; Khalil, I. Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Prim. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehlings, M.G.; Wilson, J.R.; Harrop, J.S.; Kwon, B.K.; Tetreault, L.A.; Arnold, P.M.; Singh, J.M.; Hawryluk, G.; Dettori, J.R. Efficacy and safety of methylprednisolone sodium succinate in acute spinal cord injury: A systematic review. Glob. Spine J. 2017, 7, 116S–137S. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Warren, P.M.; Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.S. Inflammation and apoptosis: Linked therapeutic targets in spinal cord injury. Trends Mol. Med. 2004, 10, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, T.M.; Burda, J.E.; Sofroniew, M.V. Cell biology of spinal cord injury and repair. J. Clin. Investig. 2017, 127, 3259–3270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lv, H.Q.; Chao, X.; Xu, W.X.; Liu, Y.; Ling, G.X.; Zhang, P. Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil. Med. Res. 2022, 9, 16. [Google Scholar] [CrossRef]
- He, Z.; Jin, Y. Intrinsic control of axon regeneration. Neuron 2016, 90, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Fan, C.X.; You, Z.F.; Xiao, Z.F.; Zhao, Y.N.; Dai, J.W. Advances in Biomaterial-Based spinal cord injury repair. Adv. Funct. Mater. 2022, 32. [Google Scholar] [CrossRef]
- Wertheim, L.; Edri, R.; Goldshmit, Y.; Kagan, T.; Noor, N.; Ruban, A.; Shapira, A.; Gat-Viks, I.; Assaf, Y.; Dvir, T. Regenerating the injured spinal cord at the chronic phase by engineered iPSCs-Derived 3D neuronal networks. Adv. Sci. 2022, 9, e2105694. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Marchini, A.; Raspa, A.; Pugliese, R.; El, M.M.; Pastori, V.; Lecchi, M.; Vescovi, A.L.; Gelain, F. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl. Acad. Sci. USA 2019, 116, 7483–7492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Chueng, S.D.; Li, Y.; Patel, M.; Rathnam, C.; Dey, G.; Wang, L.; Cai, L.; Lee, K.B. A biodegradable hybrid inorganic nanoscaffold for advanced stem cell therapy. Nat. Commun. 2018, 9, 3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandai, M.; Kurimoto, Y.; Takahashi, M. Autologous induced Stem-Cell-Derived retinal cells for macular degeneration. N. Engl. J. Med. 2017, 377, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, Z.N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Akle, C.A.; Adinolfi, M.; Welsh, K.I.; Leibowitz, S.; Mccoll, I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet 1981, 2, 1003–1005. [Google Scholar] [CrossRef]
- Li, J.; Qiu, C.; Wei, Y.; Yuan, W.; Liu, J.; Cui, W.; Zhou, J.; Qiu, C.; Guo, L.; Huang, L.; et al. Human amniotic epithelial stem Cell-Derived retinal pigment epithelium cells repair retinal degeneration. Front. Cell Dev. Biol. 2021, 9, 737242. [Google Scholar] [CrossRef]
- Yang, P.J.; Yuan, W.X.; Liu, J.; Li, J.Y.; Tan, B.; Qiu, C.; Zhu, X.L.; Qiu, C.; Lai, D.M.; Guo, L.H.; et al. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol. Sin. 2018, 39, 1305–1316. [Google Scholar] [CrossRef]
- Miki, T.; Strom, S.C. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006, 2, 133–142. [Google Scholar] [CrossRef]
- Miki, T.; Grubbs, B. Therapeutic potential of placenta-derived stem cells for liver diseases: Current status and perspectives. J. Obstet. Gynaecol. Res. 2014, 40, 360–368. [Google Scholar] [CrossRef]
- Miki, T. A rational strategy for the use of amniotic epithelial stem cell therapy for liver diseases. Stem Cells Transl. Med. 2016, 5, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Qiu, C.; Zhang, Z.; Yuan, W.; Ge, Z.; Tan, B.; Yang, P.; Liu, J.; Zhu, X.; Qiu, C.; et al. Subretinal transplantation of human amniotic epithelial cells in the treatment of autoimmune uveitis in rats. Cell Transplant. 2018, 27, 1504–1514. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Yuan, W.; Li, J.; Yang, P.; Ge, Z.; Liu, J.; Qiu, C.; Zhu, X.; Qiu, C.; Lai, D.; et al. Therapeutic effect of human amniotic epithelial cells in murine models of Hashimoto’s thyroiditis and Systemic lupus erythematosus. Cytotherapy 2018, 20, 1247–1258. [Google Scholar] [CrossRef]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.; Wu, J.; Mu, J.; Gao, J. Biomaterials reinforced MSCs transplantation for spinal cord injury repair. Asian J. Pharm. Sci. 2022, 17, 4–19. [Google Scholar] [CrossRef]
- Xie, M.J.; Zheng, Y.T.; Gao, Q.; He, Y. Facile 3D cell culture protocol based on photocurable hydrogels. Bio-Des. Manuf. 2021, 4, 149–153. [Google Scholar] [CrossRef]
- Van Den Bulcke, A.I.; Bogdanov, B.; De Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, R.Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Chen, Y.; Liu, R.; Liu, X.; Liu, C.; Shao, Z.; Xiong, L.; Liu, X.; Sun, W.; Mi, S. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat. Commun. 2020, 11, 1435. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yu, K.; Gao, Q.; He, Y. Projection-based 3D bioprinting for hydrogel scaffold manufacturing. Bio-Des. Manuf. 2022, 5, 633–639. [Google Scholar] [CrossRef]
- Gao, M.; Lu, P.; Bednark, B.; Lynam, D.; Conner, J.M.; Sakamoto, J.; Tuszynski, M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 2013, 34, 1529–1536. [Google Scholar] [CrossRef] [Green Version]
- Koffler, J.; Samara, R.F.; Rosenzweig, E.S. Using templated agarose scaffolds to promote axon regeneration through sites of spinal cord injury. Methods Mol. Biol. 2014, 1162, 157–165. [Google Scholar]
- Chen, B.; Li, Y.; Yu, B.; Zhang, Z.; Brommer, B.; Williams, P.R.; Liu, Y.; Hegarty, S.V.; Zhou, S.; Zhu, J.; et al. Reactivation of dormant relay pathways in injured spinal cord by KCC2 manipulations. Cell 2018, 174, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp. Neurol. 1996, 139, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Piltti, K.M.; Salazar, D.L.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Safety of epicenter versus intact parenchyma as a transplantation site for human neural stem cells for spinal cord injury therapy. Stem Cells Transl. Med. 2013, 2, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Qiu, C.; Ge, Z.; Cui, W.; Yu, L.; Li, J. Human amniotic epithelial stem cells: A promising seed cell for clinical applications. Int. J. Mol. Sci. 2020, 21, 7730. [Google Scholar] [CrossRef] [PubMed]
- Miki, T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am. J. Reprod. Immunol. 2018, 80, e13003. [Google Scholar] [CrossRef] [PubMed]
- Vodyanik, M.A.; Yu, J.; Zhang, X.; Tian, S.; Stewart, R.; Thomson, J.A.; Slukvin, I.I. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 2010, 7, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Slukvin, I.I.; Kumar, A. The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell. Mol. Life Sci. 2018, 75, 3507–3520. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Patel, J.; Hutmacher, D.W.; Fisk, N.M.; Khosrotehrani, K. Meso-Endothelial bipotent progenitors from human placenta display distinct molecular and cellular identity. Stem Cell Rep. 2018, 10, 890–904. [Google Scholar] [CrossRef] [Green Version]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015, 33, 1962–1974. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, C.; Sun, Y.; Li, J.; Xu, Y.; Zhou, J.; Qiu, C.; Zhang, S.; He, Y.; Yu, L. Therapeutic Effect of Biomimetic Scaffold Loaded with Human Amniotic Epithelial Cell-Derived Neural-like Cells for Spinal Cord Injury. Bioengineering 2022, 9, 535. https://doi.org/10.3390/bioengineering9100535
Qiu C, Sun Y, Li J, Xu Y, Zhou J, Qiu C, Zhang S, He Y, Yu L. Therapeutic Effect of Biomimetic Scaffold Loaded with Human Amniotic Epithelial Cell-Derived Neural-like Cells for Spinal Cord Injury. Bioengineering. 2022; 9(10):535. https://doi.org/10.3390/bioengineering9100535
Chicago/Turabian StyleQiu, Chen, Yuan Sun, Jinying Li, Yuchen Xu, Jiayi Zhou, Cong Qiu, Shaomin Zhang, Yong He, and Luyang Yu. 2022. "Therapeutic Effect of Biomimetic Scaffold Loaded with Human Amniotic Epithelial Cell-Derived Neural-like Cells for Spinal Cord Injury" Bioengineering 9, no. 10: 535. https://doi.org/10.3390/bioengineering9100535
APA StyleQiu, C., Sun, Y., Li, J., Xu, Y., Zhou, J., Qiu, C., Zhang, S., He, Y., & Yu, L. (2022). Therapeutic Effect of Biomimetic Scaffold Loaded with Human Amniotic Epithelial Cell-Derived Neural-like Cells for Spinal Cord Injury. Bioengineering, 9(10), 535. https://doi.org/10.3390/bioengineering9100535