The Effect of Concave-Side Intertransverse Ligament Laxity on the Stress of AIS Lumbar Spine Based on Finite Element Method
Abstract
:Simple Summary
Abstract
1. Introduction
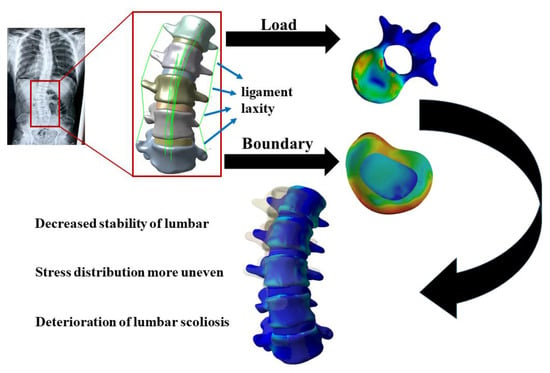
2. Materials and Methods
2.1. Data Acquisition
2.2. Establishment of the Model
2.3. Material Parameters
2.4. Boundary and Loading Conditions
2.5. Model Validation
3. Results
3.1. Established Model
3.2. Validation Results
3.3. Lumbar Range of Motion
3.4. Vertebral Stress
3.5. Intervertebral Disc Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.Q.; Zhao, Y.F.; Bai, Y.S.; Liu, K.P.; He, S.S.; Wang, C.F.; Zhang, J.T.; Li, M. Factors affecting curve flexibility in skeletally immature and mature idiopathic scoliosis. J. Orthop. Sci. 2011, 16, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.; Gibson, A.; Dannawi, Z.; Noordeen, H. Adolescent idiopathic scoliosis. BMJ 2013, 346, f2508. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wang, S.R.; Qiu, G.X.; Zhang, J.G.; Zhuang, Q.Y. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin. Med. J. 2020, 133, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Kurutz, M.; Oroszváry, L. Finite element analysis of weightbath hydrotraction treatment of degenerated lumbar spine segments in elastic phase. J. Biomech. 2010, 43, 433–441. [Google Scholar] [CrossRef]
- Ghayem-Hasankhani, E.; Omidi-Kashani, F. Generalized Ligamentous Laxity; a Parameter Should not to be Forgotten in Preoperative Planning of Adolescent Idiopathic Scoliosis. Iran. Red Crescent Med. J. 2012, 14, 702–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mabrouk, M.S.; Marzouk, S.Y.; Afify, H.M. A biomechanical analysis of prosthesis disc in lumbar spinal segment using three-dimensional finite element modelling. Int. J. Biomed. Eng. Technol. 2022, 39, 1–21. [Google Scholar] [CrossRef]
- Kalichman, L.; Kendelker, L.; Bezalel, T. Bracing and exercise-based treatment for idiopathic scoliosis. J. Bodyw. Mov. Ther. 2016, 20, 56–64. [Google Scholar] [CrossRef]
- Mehlman, C.T.; Araghi, A.; Roy, D.R. Hyphenated history: The Hueter-Volkmann law. Am. J. Orthop. 1997, 26, 798–800. [Google Scholar]
- McCullen, G.M.; Garfin, S.R. Spine update: Cervical spine internal fixation using screw and screw-plate constructs. Spine 2000, 25, 643–652. [Google Scholar] [CrossRef]
- Bogduk, N. Functional anatomy of the spine. Handb. Clin. Neurol. 2016, 136, 675–688. [Google Scholar] [CrossRef]
- Burwell, R.G.; Dangerfield, P.H.; Freeman, B.J. Concepts on the pathogenesis of adolescent idiopathic scoliosis. Bone growth and mass, vertebral column, spinal cord, brain, skull, extra-spinal left-right skeletal length asymmetries, disproportions and molecular pathogenesis. Stud. Health Technol. Inform. 2008, 135, 3–52. [Google Scholar] [PubMed]
- Shi, L.; Wang, D.; Driscoll, M.; Villemure, I.; Chu, W.C.; Cheng, J.C.; Aubin, C.E. Biomechanical analysis and modeling of different vertebral growth patterns in adolescent idiopathic scoliosis and healthy subjects. Scoliosis 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, R.C.; Schlösser, T.P.C.; Colo, D.; Vavruch, L.; van Stralen, M.; Vincken, K.L.; Malmqvist, M.; Kruyt, M.C.; Tropp, H.; Castelein, R.M. Anterior spinal overgrowth is the result of the scoliotic mechanism and is located in the disc. Spine 2017, 42, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Shi, Y.; Xu, B.; Yang, Z. The uncoupled anterior and posterior spinal ligament tension (UAPLT)—An improvement to three-dimensional spring model of adolescent idiopathic scoliosis (AIS) pathogenesis. Med. Hypothes. 2021, 152, 110616. [Google Scholar] [CrossRef] [PubMed]
- Hadley-Miller, N.; Mims, B.; Milewicz, D.M. The potential role of the elastic fiber system in adolescent idiopathic scoliosis. J. Bone Jt. Surg. Am. Vol. 1994, 76, 1193–1206. [Google Scholar] [CrossRef]
- Imai, K. Computed tomography-based finite element analysis to assess fracture risk and osteoporosis treatment. World J. Exp. Med. 2015, 5, 182. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Huang, J.; Teo, E.C.; Gu, Y. Effect of displacement degree of distal chevron osteotomy on metatarsal stress: A finite element method. Biology 2022, 11, 127. [Google Scholar] [CrossRef]
- Belytschko, T.B.; Andriacchi, T.P.; Schultz, A.B.; Galante, J.O. Analog studies of forces in the human spine: Computational techniques. J. Biomech. 1973, 6, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Wong, D.W.-C.; Chen, T.L.-W.; Chen, S.F.; Zhang, G.; Tan, Q.; Zhang, M. Different design feature combinations of flatfoot orthosis on plantar fascia strain and plantar pressure: A muscle-driven finite element analysis with taguchi method. Front. Bioeng. Biotechnol. 2022, 10, 853085. [Google Scholar] [CrossRef]
- Zhang, Q.; Chon, T.; Zhang, Y.; Baker, J.S.; Gu, Y. Finite element analysis of the lumbar spine in adolescent idiopathic scoliosis subjected to different loads. Comput. Biol. Med. 2021, 136, 104745. [Google Scholar] [CrossRef]
- Kamal, Z.; Rouhi, G. Stress distribution changes in growth plates of a trunk with adolescent idiopathic scoliosis following unilateral muscle paralysis: A hybrid musculoskeletal and finite element model. J. Biomech. 2020, 111, 109997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, Y.; Chon, T.E.; Baker, J.S.; Gu, Y. Analysis of stress and stabilization in adolescent with osteoporotic idiopathic scoliosis: Finite element method. Comput. Methods Biomech. Biomed. Eng. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Izatt, M.T.; Labrom, R.D.; Askin, G.N.; Adam, C.J. An FE investigation simulating intra-operative corrective forces applied to correct scoliosis deformity. Scoliosis 2013, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, S.; Xue, B.; Xiong, Y. Three-Dimensional Biomechanical Finite Element Analysis of Lumbar Disc Herniation in Middle Aged and Elderly. J. Healthc. Eng. 2022, 2022, 7107702. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, H.; Amling, M.; Pösl, M.; Hahn, M.; Delling, G. The thickness of human vertebral cortical bone and its changes in aging and osteoporosis: A histomorphometric analysis of the complete spinal column from thirty-seven autopsy specimens. J. Bone Miner. Res. 1997, 12, 89–95. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Y.; Lou, S.; Zhang, D.; Liao, S. Construction and validation of a three-dimensional finite element model of degenerative scoliosis. J. Orthop. Surg. Res. 2015, 10, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.C.; Wilcox, R.K. Finite element analysis of the spine: Towards a framework of verification, validation and sensitivity analysis. Med. Eng. Phys. 2008, 30, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-S.; Hu, H.-T.; Lin, R.-M.; Huang, K.-Y.; Lin, P.-C.; Zhong, Z.-C.; Hseih, M.-L. Biomechanical analysis of the lumbar spine on facet joint force and intradiscal pressure—A finite element study. BMC Musculoskelet. Disord. 2010, 11, 151. [Google Scholar] [CrossRef] [Green Version]
- Polikeit, A.; Ferguson, S.J.; Nolte, L.P.; Orr, T.E. Factors influencing stresses in the lumbar spine after the insertion of intervertebral cages: Finite element analysis. Eur. Spine J. 2003, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Sylvestre, P.-L.; Villemure, I.; Aubin, C.-E. Finite element modeling of the growth plate in a detailed spine model. Med. Biol. Eng. Comput. 2007, 45, 977–988. [Google Scholar] [CrossRef]
- Pintar, F.A.; Yoganandan, N.; Myers, T.; Elhagediab, A.; Sances, A., Jr. Biomechanical properties of human lumbar spine ligaments. J. Biomech. 1992, 25, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Fortuna, R.; Vaz, M.A.; Youssef, A.R.; Longino, D.; Herzog, W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J. Biomech. 2011, 44, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Koganezawa, M. Genes and circuits of courtship behaviour in Drosophila males. Nat. Rev. Neurosci. 2013, 14, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Chun, H.-J.; Kang, K.-T.; Lee, H.-M.; Kim, H.-S.; Moon, E.-S.; Park, J.-O.; Hwang, B.-H.; Son, J.-H.; Moon, S.-H. A validated finite element analysis of nerve root stress in degenerative lumbar scoliosis. Med. Biol. Eng. Comput. 2009, 47, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.K.; Beheshtiha, A.S.; van der Meer, R.; Willems, P.C.; Arts, J.J.; Ito, K.; van Rietbergen, B. Validation of a finite element model of the thoracolumbar spine to study instrumentation level variations in early onset scoliosis correction. J. Mech. Behav. Biomed. Mater. 2021, 117, 104360. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.M.; Mazzoleni, A.P.; Hey, L.A. Development and validation of a computationally efficient finite element model of the human lumbar spine: Application to disc degeneration. Int. J. Spine Surg. 2020, 14, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Tsouknidas, A.; Michailidis, N.; Savvakis, S.; Anagnostidis, K.; Bouzakis, K.-D.; Kapetanos, G. A finite element model technique to determine the mechanical response of a lumbar spine segment under complex loads. J. Appl. Biomech. 2012, 28, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heth, J.A.; Hitchon, P.W.; Goel, V.K.; Rogge, T.N.; Drake, J.S.; Torner, J.C. A biomechanical comparison between anterior and transverse interbody fusion cages. Spine 2001, 26, e261–e267. [Google Scholar] [CrossRef]
- Gu, Y.D.; Li, J.S.; Lake, M.J.; Zeng, Y.J.; Ren, X.J.; Li, Z.Y. Image-based midsole insert design and the material effects on heel plantar pressure distribution during simulated walking loads. Comput Methods Biomech Biomed Eng. 2011, 14, 747–753. [Google Scholar] [CrossRef]
- Zhang, Y.; Awrejcewicz, J.; Szymanowska, O.; Shen, S.; Zhao, X.; Baker, J.S.; Gu, Y. Effects of severe hallux valgus on metatarsal stress and the metatarsophalangeal loading during balanced standing: A finite element analysis. Comput. Biol. Med. 2018, 97, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Zhang, Q.; Song, Y.; Hu, Q.; Fekete, G.; Baker, J.S.; Gu, Y. Biomechanical characteristics of 2 different posterior fixation methods of bilateral pedicle screws: A finite element analysis. Medicine 2022, 101, e30419. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, Y.; Cai, Z.; Wang, W.; Xia, Y.; Zheng, G.; Liang, Y.; Wang, Y. Effect of osteoporosis on internal fixation after spinal osteotomy: A finite element analysis. Clin. Biomech. 2019, 69, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, E.; Singh, A.; Hachem, B.; Benoit, D.; Rustagi, R.; Vithoulkas, G.; Mac-Thiong, J.M.; Syed, H. Finite element assessment of a disc-replacement implant for treating scoliotic deformity. Clin. Biomech. 2021, 84, 105326. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.M., Jr.; Smith, M.D. Thoracic insufficiency syndrome and exotic scoliosis. J. Bone Jt. Surg. Am. Vol. 2007, 89 (Suppl. S1), 108–122. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, Z.; Cen, X.; Thirupathi, A.; Sun, D.; Gu, Y. The influence of different rope jumping methods on adolescents’ lower limb biomechanics during the ground-contact phase. Children 2022, 9, 721. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, S.P.J.; Tromp, I.N.; Yazici, M.; Kempen, D.H.R.; Castelein, R.M.; Kruyt, M.C. A comparison of growth among growth-friendly systems for scoliosis: A systematic review. Spine J. 2019, 19, 789–799. [Google Scholar] [CrossRef]
- Luque, E.R. Paralytic scoliosis in growing children. Clin. Orthop. Relat. Res. 1982, 163, 202–209. [Google Scholar] [CrossRef]
- Thakre, P.S.; Zakiuddin, K.S.; Khan, I.A.; Faizan, M.S. Finite element analysis of tibia bone. Int. J. Biomed. Eng. Technol. 2021, 35, 318–339. [Google Scholar] [CrossRef]
- Ying, J.; Cen, X.; Yu, P. Effects of Eccentric Exercise on Skeletal Muscle Injury: From An Ultrastructure Aspect: A Review. Phys. Act. Health 2021, 5, 15–20. [Google Scholar] [CrossRef]
- Stokes, I.A. Mechanical modulation of spinal growth and progression of adolescent scoliosis. Stud. Health Technol. Inform. 2008, 135, 75–83. [Google Scholar]
- Goto, M.; Kawakami, N.; Azegami, H.; Matsuyama, Y.; Takeuchi, K.; Sasaoka, R. Buckling and bone modeling as factors in the development of idiopathic scoliosis. Spine 2003, 28, 364–370. [Google Scholar] [CrossRef]
- MacEwen, G.D.; Bunnell, W.P.; Sriram, K. Acute neurological complications in the treatment of scoliosis. A report of the Scoliosis Research Society. J. Bone Jt. Surg. Am. 1975, 57, 404–408. [Google Scholar] [CrossRef]
- Kamerlink, J.R.; Errico, T.; Xavier, S.; Patel, A.; Patel, A.; Cohen, A.; Reiger, M.; Dryer, J.; Feldman, D.; Lonner, B.; et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine 2010, 35, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Ruopsa, N.; Vastamäki, H.; Ristolainen, L.; Vastamäki, M.; Saltychev, M. Convergent Validity of Thoracic Outlet Syndrome Index (TOSI). Phys. Act. Health 2022, 6, 16–25. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, H.; Jiang, X.; Li, S.; Zhang, Q.; Baker, J.S.; Gu, Y. New insights for the design of bionic robots: Adaptive motion adjustment strategies during feline landings. Front. Vet. Sci. 2022, 9, 836043. [Google Scholar] [CrossRef] [PubMed]
- Bucolo, M.; Buscarino, A.; Famoso, C.; Fortuna, L.; Gagliano, S. Imperfections in integrated devices allow the emergence of unexpected strange attractors in electronic circuits. IEEE Access 2021, 9, 29573–29583. [Google Scholar] [CrossRef]





| Component | Yang’s Modulus E (MPa) | Poisson’s Ratio (v) | Mesh Size (mm) |
|---|---|---|---|
| Cortical bone | 12,000 | 0.30 | 0.5 |
| Cancellous bone | 100 | 0.30 | 1 |
| Endplate | 25 | 0.25 | 0.5 |
| Annulus fibrosis | 4.2 | 0.45 | 1 |
| Articular cartilage | 50 | 0.30 | 0.5 |
| Nucleus pulposus | 1 | 0.50 | 1 |
| Ligament | Young’s Modulus (MPa) | Cross-Sectional Area (mm2) | Average Length (mm) |
|---|---|---|---|
| Anterior longitudinal ligament | 7.8 | 22.4 | 20 |
| Posterior longitudinal ligament | 10.0 | 7.0 | 12 |
| Ligamentum flavum | 17.0 | 14.1 | 15 |
| Interspinous ligament | 10.0 | 0.6 | 32 |
| Capsular ligament | 7.5 | 10.5 | 5 |
| Supraspinous ligament | 8.0 | 10.5 | 11 |
| Intertransverse ligament | 10.0 | 14.1 | 13 |
| Relaxation of intertransverse ligament | 5.0 | 14.1 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, Q.; Zhang, Y.; Arthur, M.; Teo, E.-C.; Bíró, I.; Gu, Y. The Effect of Concave-Side Intertransverse Ligament Laxity on the Stress of AIS Lumbar Spine Based on Finite Element Method. Bioengineering 2022, 9, 724. https://doi.org/10.3390/bioengineering9120724
Zhang L, Zhang Q, Zhang Y, Arthur M, Teo E-C, Bíró I, Gu Y. The Effect of Concave-Side Intertransverse Ligament Laxity on the Stress of AIS Lumbar Spine Based on Finite Element Method. Bioengineering. 2022; 9(12):724. https://doi.org/10.3390/bioengineering9120724
Chicago/Turabian StyleZhang, Linjie, Qiaolin Zhang, Yan Zhang, Musinguzi Arthur, Ee-Chon Teo, István Bíró, and Yaodong Gu. 2022. "The Effect of Concave-Side Intertransverse Ligament Laxity on the Stress of AIS Lumbar Spine Based on Finite Element Method" Bioengineering 9, no. 12: 724. https://doi.org/10.3390/bioengineering9120724
APA StyleZhang, L., Zhang, Q., Zhang, Y., Arthur, M., Teo, E. -C., Bíró, I., & Gu, Y. (2022). The Effect of Concave-Side Intertransverse Ligament Laxity on the Stress of AIS Lumbar Spine Based on Finite Element Method. Bioengineering, 9(12), 724. https://doi.org/10.3390/bioengineering9120724