Development of Novel Lipid-Based Formulations for Water-Soluble Vitamin C versus Fat-Soluble Vitamin D3
Abstract
:1. Introduction
2. Materials and Methods
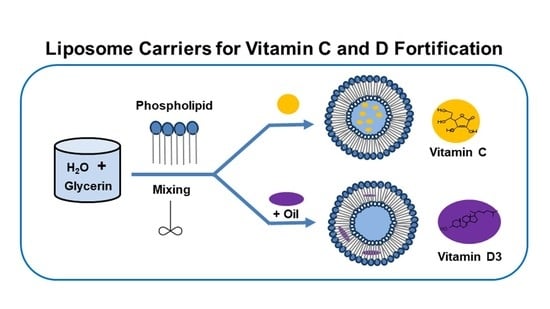
2.1. Preparation of Liposomal Vitamin C
2.2. Preparation of Liposomal Vitamin D3
2.3. Characterization of Vitamin C and Vitamin D3 in Liposome Forms
2.3.1. Transmission Electron Microscopy (TEM) Analysis
2.3.2. Particle Size Distribution and Zeta Potential
2.3.3. Encapsulation Efficiency (EE%) and Vitamins Loading (VL%)
2.3.4. Instrumentation and Chromatographic Conditions
2.3.5. Sample Preparation for UHPLC Analysis
2.3.6. Liposomal Stability
3. Results and Discussion
3.1. Optimization of Formulation
3.2. Morphology by TEM
3.3. Size and Zeta Potential
3.4. Evaluation of Encapsulation Efficiency (EE%) and Vitamin Loading (VL%)
3.5. Determination of Vitamin C Potency in Liposomes
3.6. Determination of Vitamin D3 Potency in Liposomes
3.7. Comparative Analysis of Vitamin Formulations
3.8. Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omri, A.; Suntres, Z.E.; Shek, P.N. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem. Pharmacol. 2002, 64, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Schiffelers, R.M.; Storm, G.; Bakker-Woudenberg, I.A. Host factors influencing the preferential localization of sterically stabilized liposomes in Klebsiella pneumoniae-infected rat lung tissue. Pharm. Res. 2001, 18, 780–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stano, P.; Bufali, S.; Pisano, C.; Bucci, F.; Barbarino, M.; Santaniello, M.; Carminati, P.; Luisi, P.L. Novel camptothecin analogue (gimatecan)-containing liposomes prepared by the ethanol injection method. J. Liposome Res. 2004, 14, 87–109. [Google Scholar] [CrossRef] [PubMed]
- Atrooz, O.M. Effects of alkylresorcinolic lipids obtained from acetonic extract of Jordanian wheat grains on liposome properties. Int. J. Biol. Chem. 2011, 5, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Benech, R.O.; Kheadr, E.E.; Laridi, R.; Lacroix, C.; Fliss, I. Inhibition of Listeria innocua in cheddar cheese by addition of nisin Z in liposomes or by in situ production in mixed culture. Appl. Environ. Microbiol. 2002, 68, 3683–3690. [Google Scholar] [CrossRef] [Green Version]
- Shehata, T.; Ogawara, K.; Higaki, K.; Kimura, T. Prolongation of residence time of liposome by surface-modification with mixture of hydrophilic polymers. Int. J. Pharm. 2008, 359, 272–279. [Google Scholar] [CrossRef]
- Johnston, M.J.; Semple, S.C.; Klimuk, S.K.; Ansell, S.; Maurer, N.; Cullis, P.R. Characterization of the drug retention and pharmacokinetic properties of liposomal nanoparticles containing dihydrosphingomyelin. Biochim. Biophys. Acta 2007, 1768, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Hofheinz, R.D.; Gnad-Vogt, S.U.; Beyer, U.; Hochhaus, A. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs 2005, 16, 691–707. [Google Scholar] [CrossRef]
- Lasic, D.D. Applications of liposomes. In Handbook of Biological Physics; Elsevier: Amsterdam, The Netherlands, 1995; pp. 491–519. [Google Scholar]
- Elizondo, E.; Moreno, E.; Cabrera, I.; Córdoba, A.; Sala, S.; Veciana, J.; Ventosa, N. Liposomes and other vesicular systems: Structural characteristics, methods of preparation, and use in nanomedicine. Prog. Mol. Biol. Transl. Sci. 2011, 104, 1–52. [Google Scholar]
- Stein, H.; Spindler, S.; Bonakdar, N.; Wang, C.; Sandoghdar, V. Production of isolated giant unilamellar vesicles under high salt concentration. Front. Physicol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Harashima, H.; Sakata, K.; Funato, K.; Kiwada, H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm. Res. 1994, 11, 402–406. [Google Scholar] [CrossRef]
- Woodle, M.C. Sterically stabilized liposome therapeutics. Adv. Drug Deliv. Rev. 1995, 16, 249–265. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, S.K.; Labhasetwar, V. Nanotech approaches to drug delivery and imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Vemuri, S.; Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: A review. Pharm. Acta Helv. 1995, 70, 95–111. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, U.S. Liposomes in drug delivery: Progress and limitations. Int. J. Pharm. 1997, 154, 123–140. [Google Scholar] [CrossRef]
- Jesorka, A.; Orwar, O. Liposomes: Technologies and analytical applications. Annu. Rev. Anal. Chem. 2008, 1, 801–832. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P.A. comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12–45. [Google Scholar] [CrossRef] [PubMed]
- Webb, C.; Khadke, S.; Schmidt, S.T.; Roces, C.B.; Forbes, N.; Berrie, G.; Perrie, Y. The Impact of Solvent Selection: Strategies to Guide the Manufacturing of Liposomes Using Microfluidics. Pharmaceutics 2019, 11, 653–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beh, C.C.; Mammucari, R.; Foster, N.R. Formation of nanocarrier systems by dense gas processing. Langmuir 2014, 30, 11046–11054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.-L.; Dastoor, P.C.; Flynn, J.; Palmer, W.; Martin, A.; Smith, D.W.; Woldu, A.; Hua, S. Advantages and limitations of current imaging techniques for characterizing liposome morphology. Front. Pharmacol. 2018, 9, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Ruozi, B.; Belletti, D.; Tombesi, A.; Tosi, G.; Bondioli, L.; Forni, F.; Vandelli, M.A. AFM, ESEM, TEM, and CLSM in liposomal characterization: A comparative study. Int. J. Nanomed. 2011, 6, 557–563. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.T.; Aguero, S.D. Phospholipids: Properties and health effects. Nutr. Hosp. 2015, 31, 76–83. [Google Scholar]
- Van Nieuwenhuyzen, W.; Szuhaj, B.F. Effects of lecithins and proteins on the stability of emulsions. Eur. J. Lipid Sci. Technol. 1998, 100, 282–291. [Google Scholar] [CrossRef]
- Gopi, S.; Balakrishnan, P. Evaluation and Clinical Comparison Studies on Liposomal and Non-Liposomal Ascorbic Acid (Vitamin C) and their Enhanced Bioavailability. J. Liposome Res. 2021, 31, 356–364. [Google Scholar] [CrossRef]
- Alvim, I.D.; Stein, M.A.; Koury, I.P.; Dantas, F.B.H.; Cruz, C.C.V. Comparison between the spray drying and spray chilling microparticles contain ascorbic acid in a baked product application. LWT–Food Sci. Technol. 2016, 65, 689–694. [Google Scholar] [CrossRef]
- Fong, S.Y.K.; Martins, S.M.; Brandl, M.; Brandl, A.B. Solid phospholipid dispersions for oral delivery of poorly soluble drugs: Investigation into celecoxib incorporation and solubility-in vitro permeability enhancement. J. Pharm. Sci. 2016, 105, 11113–11123. [Google Scholar] [CrossRef] [PubMed]
- Matos, F.E., Jr.; Sabatino, M.D.; Passerini, N.; Favaro, T.C.S.; Albertini, B. Development and characterization of solid lipid microparticles loaded with ascorbic acid and produced by spray congealing. Food Res. Int. 2015, 67, 52–59. [Google Scholar] [CrossRef]
- Herbig, A.L.; Renard, C.M. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Paris, H.L.; Beals, J.W.; Binns, S.E.; Giordano, G.R.; Scalzo, R.L.; Schweder, M.M.; Blair, E.; Bell, C. Liposomal encapsulated ascorbic acid: Influence on vitamin C bioavailability and capacity to protect against ischemia-reperfusion injury. Nutr. Metab. Insights 2016, 9, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Parhizkar, E.; Rashedinia, M.; Karimi, M.; Alipour, S. Design and development of vitamin C-encapsulated proliposome with improved in-vitro and ex-vivo antioxidant efficacy. J. Microencapsul. 2018, 35, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Wechtersbach, L.; Poklar Ulrih, N.; Cigic, B. Liposomal stabilization of ascorbic acid in model systems and in food matrices. LWT-Food Sci. Technol. 2012, 45, 43–49. [Google Scholar] [CrossRef]
- Hickey, S.; Roberts, H.J.; Miller, N.J. Pharmacokinetics of oral vitamin C. J. Nutr. Environ. Med. 2008, 17, 169–177. [Google Scholar] [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Pastoriza-Gallego, M.J.; Losada-Barreiro, S.; Bravo-Diıaz, C. Effects of acidity and emulsifier concentration on the distribution of vitamin C in a model food emulsion. J. Phys. Org. Chem. 2012, 25, 908–915. [Google Scholar] [CrossRef]
- Turner, J.V.; Agatonovic-Kustrin, S. In Silico Prediction of Oral Bioavailability. In Comprehensive Medicinal Chemistry II, 1st ed.; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 699–724. [Google Scholar]
- Comunian, T.A.; Abbaspourrad, A.; Favaro, T.C.S.; Weitz, D.A. Fabrication of solid lipid microcapsules containing ascorbic acid using a microfluidic technique. Food Chem. 2014, 152, 271–275. [Google Scholar] [CrossRef]
- Farhang, B.; Kakuda, Y.; Corredig, M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci. Technol. 2012, 92, 353–366. [Google Scholar] [CrossRef]
- Chakraborty, A.; Jana, N.R. Vitamin C-Conjugated nanoparticle protects cells from oxidative stress at low doses but induces oxidative stress and cell death at high doses. ACS Appl. Mater. Interfaces 2017, 9, 41807–41817. [Google Scholar] [CrossRef]
- Blesso, C.N. Egg phospholipids and cardiovascular health. Nutrients 2015, 7, 2731–2747. [Google Scholar] [CrossRef] [Green Version]
- Kullenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A.; Jacob, J.; Kalarikkal, N.; Thomas, S.; Guo, Q. Preparation, characterization and in vitro study of liposomal curcumin powder by cost effective nanofiber weaving technology. New J. Chem. 2018, 42, 5117–5127. [Google Scholar] [CrossRef]
- Norman, A.W.; Henry, H.L. Vitamin D. In Handbook of Vitamins, 4th ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Bender, D.A. Nutritional Biochemistry of the Vitamins; Cambridge University Press: New York, NY, USA, 2003. [Google Scholar]
- Carlberg, C. Nutrigenomics of vitamin D. Nutrients 2019, 11, 676–691. [Google Scholar] [CrossRef] [Green Version]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and novel actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef]
- Livney, Y.D.; Semo, E.; Danino, D.; Kesselman, E. Nanoencapsulation of Hydrophobic Nutraceutical Substances within Casein Micelles. In Proceedings of the XIVth International Workshop on Bioencapsulation, Lausanne, Switzerland, 5–7 October 2006; pp. 1–4. [Google Scholar]
- Wilson, L.R.; Tripkovic, L.; Hart, K.H.; Lanham-New, S.A. Vitamin D deficiency as a public health issue: Using vitamin D2 or vitamin D3 in future fortification strategies. Proc. Nutr. Soc. 2017, 76, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Corthésy, B.; Bioley, G. Lipid-based particles: Versatile delivery systems for mucosal vaccination against infection. Front. Immunol. 2018, 9, 431–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Bellissimo, N.; Rousseau, D. The physical state of emulsified edible oil modulates its in vitro digestion. J. Agric. Food Chem. 2017, 65, 9120–9127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Behera, C.; Paudwal, G.; Rawat, N.; Baldi, A.; Gupta, P.N. Recent advances in formulation strategies for efficient delivery of vitamin D. AAPS Pharm. Sci. Tech. 2019, 20, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Maia Campos, P.M.B.; De Camargo Junior, F.B.; De Andrade, J.P.; Gaspar, L.R. Efficacy of cosmetic formulations containing dispersion of liposome with magnesium ascorbyl phosphate, -lipoic acid and kinetin. Photochem. Photobiol. 2012, 88, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Lin, M.; Gu, Y.; Zhao, X.; Hu, G. Preparation of PEG-modified proanthocyanidin liposome and its application in cosmetics. Eur. Food Res. Technol. 2015, 240, 1013–1021. [Google Scholar] [CrossRef]
- Kirby, B.J.; Hasselbrink, E.F., Jr. Zeta Potential of Microfluidic Substrates: 1. Theory, Experimental Techniques, and Effects on Separations. Electrophoresis 2004, 25, 187–202. [Google Scholar] [CrossRef]
- Maione-Silva, L.; De Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522–536. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of Nanoliposomal Vitamin D3 for Potential Application in Beverage Fortification. Adv. Pharm. Bull 2014, 4, 569–575. [Google Scholar]
- Afrooz, H.; Ahmadi, F.; Fallahzadeh, F.; Mousavi-Fard, H.; Alipour, S. Design and characterization of paclitaxel–verapamil co-encapsulated PLGA nanoparticles: Potential system for overcoming P-glycoprotein mediated MDR. J. Drug Deliv. Sci. Technol. 2017, 41, 174–181. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Amini, A.; Akbari-Javar, H.; Baha’addini Beigi Zarandi, B.F.; Montaseri, H.; Dinarvand, R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. Daru 2018, 26, 165–177. [Google Scholar] [CrossRef]
- Jeung, J.J. Vitamin C Delivery System and Liposomal Composition Thereof. U.S. Patent 0367480 A1, 22 December 2016. [Google Scholar]
- Cipolla, D.; Wu, H.; Gonda, I.; Eastman, S.; Redelmeier, T.; Chan, H.-K. Modifying the Release Properties of Liposomes Toward Personalized Medicine. Pharm. Nanotechnol. 2014, 103, 1851–1862. [Google Scholar] [CrossRef] [Green Version]
- Bochot, A.; Fattal, E. Liposomes for intravitreal drug delivery: A state of the art. J. Control. Release 2012, 161, 628–634. [Google Scholar] [CrossRef]
- Moghimipour, E.; Rezaei, M.; Kouchak, M.; Ramezani, Z.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A.; Handali, S. A mechanistic study of the effect of transferrin conjugation on cytotoxicity of targeted liposomes. J. Microencapsul. 2018, 35, 548–558. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Kalyane, D.; Youngren-Ortiz, S.R.; Chougule, M.B.; Tekade, R.K. Importance of Physicochemical Characterization of Nanoparticles in Pharmaceutical Product Development Basic Fundamentals of Drug Delivery, 1st ed.; Tekade, R.K., Ed.; Academic Press: London, UK, 2019; pp. 369–400. [Google Scholar]
- Jacob, J.; Sukumaran, N.P.; Jude, S. Fiber-Reinforced-Phospholipid Vehicle-Based Delivery of L-Ascorbic Acid: Development, Characterization, ADMET Profiling, and Efficacy by a Randomized, Single-Dose, Crossover Oral Bioavailability Study. ACS Omega 2021, 6, 5560–5568. [Google Scholar] [CrossRef]
- Samimi, S.; Maghsoudnia, N.; Eftekhari, R.B.; Dorkoosh, F. Lipid-Based Nanoparticles for Drug Delivery Systems. In Characterization and Biology of Nanomaterials for Drug Delivery, 1st ed.; Mohapatra, S., Ranjan, R., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–76. [Google Scholar]
- Joseph, E.; Singhvi, G. Multifunctional Nanocrystals for Cancer Therapy: A Potential Nanocarrier. In Nanomaterials for Drug Delivery and Therapy, 1st ed.; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 91–116. [Google Scholar]
- Soema, P.C.; Willems, G.J.; Amorij, J.-P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Prantl, L.; Eigenberger, A.; Gehmert, S.; Haerteis, S.; Aung, T.; Rachel, R.; Jung, E.M.; Felthaus, O. Enhanced resorption of liposomal packed vitamin C monitored by ultrasound. J. Clin. Med. 2020, 9, 1616–1628. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Betageri, G.V.; Singh, M. Factors affecting microencapsulation of drugs in liposomes. J. Microencapsul. 1995, 12, 229–246. [Google Scholar] [CrossRef]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New oral liposomal vitamin C formulation: Properties and bioavailability. J. Liposome Res. 2019, 30, 227–234. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F.; Chen, L. Encapsulation of anthocyanin in liposomes using supercritical carbon dioxide: Effects of anthocyanin and sterol concentrations. J. Funct. Foods 2017, 34, 159–167. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Gachumi, G.; Wasan, K.M.; Dallal Bashi, Z.; El-Aneed, A.; Badea, I. Development and Characterization of Liposomal Formulations Containing Phytosterols Extracted from Canola Oil Deodorizer Distillate along with Tocopherols as Food Additives. Pharmaceutics 2019, 11, 185–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsanasco, M.; Piotrkowski, B.; Calabró, V.; Alonso, S.; Chiaramoni, N. Bioactive constituents in liposomes incorporated in orange juice as new functional food: Thermal stability, rheological and organoleptic properties. J. Food Sci. Technol. 2015, 52, 7828–7838. [Google Scholar] [CrossRef] [PubMed]
- Isailović, B.D.; Kostić, I.T.; Zvonar, A.; Đorđević, V.B.; Gašperlin, M.; Nedović, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef] [Green Version]
- Kiani, A.; Fathi, M.; Ghasemi, S.M. Production of novel vitamin D3 loaded lipid nanocapsules for milk fortification. Int. J. Food Prop. 2017, 20, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Yan, Z.; Duan, M. Characterisation and Skin Distribution of Lecithin-Based Coenzyme Q10-Loaded Lipid Nanocapsules. Nanoscale Res. Lett. 2010, 5, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Dałek, P.; Drabik, D.; Wołczańska, H.; Foryś, A.; Jagas, M.; Jędruchniewicz, N.; Przybyło, M.; Witkiewicz, W.; Langner, M. Bioavailability by design-Vitamin D3 liposomal delivery vehicles. Nanomed. Nanotechnol. Boil. Med. 2022, 43, 102552–102563. [Google Scholar] [CrossRef]
- Bonnekoh, B.; Roding, J.; Krueger, G.R.; Ghyczy, M.; Mahrle, G. Increase of lipid fluidity and suppression of proliferation resulting from liposome uptake by human keratinocytes in vitro. Br. J. Dermatol. 1991, 124, 333–340. [Google Scholar] [CrossRef]
- White, P.J.; Fogarty, R.D.; McKean, S.C.; Venables, D.J.; Werther, G.A.; Wraight, C.J. Oligonucleotide uptake in cultured keratinocytes: Influence of confluence, cationic liposomes, and keratinocyte cell type. J. Infect. Dis. 1999, 112, 699–705. [Google Scholar] [CrossRef]




| Encapsulation Efficiency (EE%) | Vitamin Loading (VL%) | |
|---|---|---|
| Vitamin C | 47.15% | 85.57% |
| Vitamin D3 | >90.00% | 78.45% |
| Vitamin C | Top | Bottom |
|---|---|---|
| Concentration | 204.50 mg/mL | 192.90 mg/mL |
| Recovery value (%) | 97.38% | 91.86% |
| Vitamin D3 | Top | Bottom |
|---|---|---|
| Concentration | 13.88 µg/mL | 13.30 µg/mL |
| Recovery value (%) | 100.95% | 96.73% |
| Vitamin C | Storage Temperature (°C) | 0 Days | 90 Days | ||
|---|---|---|---|---|---|
| Top | Bottom | Top | Bottom | ||
| Concentration | 4 | 204.50 mg/mL | 192.90 mg/mL | 198.79 mg/mL | 178.35 mg/mL |
| 25 | 204.50 mg/mL | 192.90 mg/mL | 175.20 mg/mL | 155.54 mg/mL | |
| Stability (%) | 4 | 102.24% | 96.42% | 99.40% | 89.17% |
| 25 | 102.24% | 96.42% | 87.60% | 77.77% | |
| Vitamin D3 | Storage Temperature (°C) | 0 Days | 90 Days | ||
|---|---|---|---|---|---|
| Top | Bottom | Top | Bottom | ||
| Concentration | 4 | 13.88 µg/mL | 13.30 µg/mL | 8.8 µg/mL | 9.8 µg/mL |
| 25 | 13.88 µg/mL | 13.30 µg/mL | 7.9 µg/mL | 8.9 µg/mL | |
| Stability (%) | 4 | 111.00% | 106.38% | 70.21% | 78.45% |
| 25 | 111.00% | 106.38% | 63.31% | 70.84% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Dehabadi, L.; Ma, Y.-C.; Wilson, L.D. Development of Novel Lipid-Based Formulations for Water-Soluble Vitamin C versus Fat-Soluble Vitamin D3. Bioengineering 2022, 9, 819. https://doi.org/10.3390/bioengineering9120819
Chen J, Dehabadi L, Ma Y-C, Wilson LD. Development of Novel Lipid-Based Formulations for Water-Soluble Vitamin C versus Fat-Soluble Vitamin D3. Bioengineering. 2022; 9(12):819. https://doi.org/10.3390/bioengineering9120819
Chicago/Turabian StyleChen, Jie, Leila Dehabadi, Yuan-Chun Ma, and Lee D. Wilson. 2022. "Development of Novel Lipid-Based Formulations for Water-Soluble Vitamin C versus Fat-Soluble Vitamin D3" Bioengineering 9, no. 12: 819. https://doi.org/10.3390/bioengineering9120819
APA StyleChen, J., Dehabadi, L., Ma, Y. -C., & Wilson, L. D. (2022). Development of Novel Lipid-Based Formulations for Water-Soluble Vitamin C versus Fat-Soluble Vitamin D3. Bioengineering, 9(12), 819. https://doi.org/10.3390/bioengineering9120819