Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. HUVEC and Hep-G2 Monolayer Cell Culture
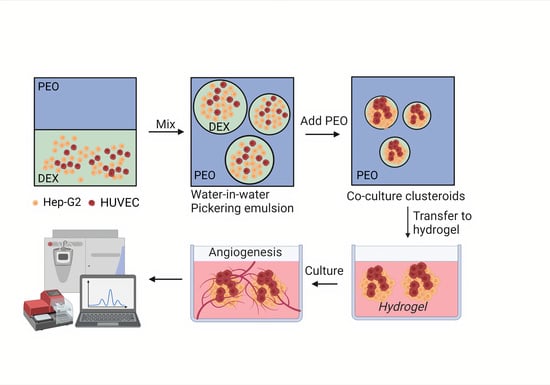
2.2.2. HUVEC and Hep-G2 3D Clusteroids Culture
2.2.3. Long-Term Growth of the Co-Culture Clusteroids in Matrigel
2.2.4. Bright-Field, Fluorescence, and Confocal Microscopy Observations
2.2.5. Spheroid Sprouts Analysis
2.2.6. HIF1-α, MMP-2, IGFBP, and VEGF ELISA
2.2.7. SEM Imaging of the Clusteroids
2.2.8. Angiogenesis Array
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Clusteroids Culture in the w/w Pickering Emulsion Template
3.2. Production of Angiogenic Factors by the Co-Cultured Clusteroids
3.3. Endothelial Cell Sprouting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Madden, L.A.; Paunov, V.N. Advanced biomedical applications based on emerging 3D cell culturing platforms. J. Mater. Chem. B Mater. Biol. Med. 2020, 8, 10487–10501. [Google Scholar] [CrossRef] [PubMed]
- Santini, M.T.; Rainaldi, G. Three-dimensional spheroid model in tumor biology. Pathobiology 1999, 67, 148–157. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Tung, Y.C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Alini, M.; Eisenstein, S.M.; Ito, K.; Little, C.; Kettler, A.A.; Masuda, K.; Melrose, J.; Ralphs, J.; Stokes, I.; Wilke, H.J. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 2008, 17, 2–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasupuleti, M.K.; Molahally, S.S.; Salwaji, S. Ethical guidelines, animal profile, various animal models used in periodontal research with alternatives and future perspectives. J. Indian Soc. Periodontol. 2016, 20, 360–368. [Google Scholar] [CrossRef]
- Glorioso, J.M.; Mao, S.A.; Rodysill, B.; Mounajjed, T.; Kremers, W.K.; Elgilani, F.; Hickey, R.D.; Haugaa, H.; Rose, C.F.; Amiot, B.; et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J. Hepatol. 2015, 63, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Izpisua Belmonte, J.C. Organoids—preclinical models of human disease. N. Engl. J. Med. 2019, 380, 569–579. [Google Scholar] [CrossRef]
- Shi, R.; Radulovich, N.; Ng, C.; Liu, N.; Notsuda, H.; Cabanero, M.; Martins-Filho, S.N.; Raghavan, V.; Li, Q.; Mer, A.S.; et al. Organoid cultures as preclinical models of non-small cell lung cancer. Clin. Cancer Res. 2020, 26, 1162–1174. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, N.I.; Hibino, N.; Nakayama, K. Principles of the Kenzan method for robotic cell spheroid-based three-dimensional bioprinting. Tissue Eng. Part B Rev. 2017, 23, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowser, D.A.; Moore, M.J. Biofabrication of neural microphysiological systems using magnetic spheroid bioprinting. Biofabrication 2019, 12, 015002. [Google Scholar] [CrossRef] [PubMed]
- Mukomoto, R.; Nashimoto, Y.; Terai, T.; Imaizumi, T.; Hiramoto, K.; Ino, K.; Yokokawa, R.; Miura, T.; Shiku, H. Oxygen consumption rate of tumour spheroids during necrotic-like core formation. Analyst. 2020, 145, 6342–6348. [Google Scholar] [CrossRef]
- Giverso, C.; Preziosi, L. Influence of the mechanical properties of the necrotic core on the growth and remodelling of tumour spheroids. Int. J. Non-Linear Mech. 2019, 108, 20–32. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002, 2, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 2005, 209–231. [Google Scholar] [CrossRef]
- Blanco, R.; Gerhardt, H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb. Perspect. Med. 2013, 3, a006569. [Google Scholar] [CrossRef]
- Rundhaug, J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005, 9, 267–285. [Google Scholar] [CrossRef]
- Sang, Q.X. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998, 8, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, H.; Betsholtz, C. How do endothelial cells orientate? EXS 2005, 3–15. [Google Scholar] [CrossRef]
- Devadas, D.; Moore, T.A.; Walji, N.; Young, E.W.K. A microfluidic mammary gland coculture model using parallel 3D lumens for studying epithelial-endothelial migration in breast cancer. Biomicrofluidics 2019, 13, 064122. [Google Scholar] [CrossRef] [PubMed]
- Patra, B.; Peng, Y.-S.; Peng, C.-C.; Liao, W.-H.; Chen, Y.-A.; Lin, K.-H.; Tung, Y.-C.; Lee, C.-H. Migration and vascular lumen formation of endothelial cells in cancer cell spheroids of various sizes. Biomicrofluidics 2014, 8, 052109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingthorsson, S.; Sigurdsson, V.; Fridriksdottir, A., Jr.; Jonasson, J.G.; Kjartansson, J.; Magnusson, M.K.; Gudjonsson, T. Endothelial cells stimulate growth of normal and cancerous breast epithelial cells in 3D culture. BMC Res. Notes 2010, 3, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.; Madden, L.A.; Paunov, V.N. High-throughput fabrication of hepatic cell clusteroids with enhanced growth and functionality for tissue engineering applications. Mater. Adv. 2020, 1, 3022–3032. [Google Scholar] [CrossRef]
- Wang, A.; Weldrick, P.J.; Madden, L.A.; Paunov, V.N. Enhanced clearing of Candida biofilms on a 3D urothelial cell in vitro model using lysozyme-functionalized fluconazole-loaded shellac nanoparticles. Biomater. Sci. 2021, 9, 6927–6939. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Weldrick, P.J.; Madden, L.A.; Paunov, V.N. Biofilm-infected human clusteroid three-dimensional coculture platform to replace animal models in testing antimicrobial nanotechnologies. ACS Appl. Mater. Interfaces 2021, 13, 22182–22194. [Google Scholar] [CrossRef] [PubMed]
- Poortinga, A.T. Microcapsules from Self-Assembled Colloidal Particles Using Aqueous Phase-Separated Polymer Solutions. Langmuir 2008, 24, 1644–1647. [Google Scholar] [CrossRef]
- Zhang, J.; Hwang, J.; Antonietti, M.; Schmidt, B.V.K.J. Water-in-Water Pickering Emulsion Stabilized by Polydopamine Particles and Cross-Linking. Biomacromolecules 2019, 20, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T.; Nicolai, T.; Benyahia, L. Stabilization of Water-in-Water Emulsions by Addition of Protein Particles. Langmuir 2013, 29, 10658–10664. [Google Scholar] [CrossRef]
- Ben Ayed, E.; Cochereau, R.; Dechancé, C.; Capron, I.; Nicolai, T.; Benyahia, L. Water-in-Water Emulsion Gels Stabilized by Cellulose Nanocrystals. Langmuir 2018, 34, 6887–6893. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, T.; Murray, B. Particle Stabilized Water in Water Emulsions. Food Hydrocoll. 2017, 68, 157–163. [Google Scholar] [CrossRef]
- Das, A.A.K.; Filby, B.W.; Geddes, D.A.; Legrande, D.; Paunov, V.N. High throughput fabrication of cell spheroids by templating water-in-water Pickering emulsions. Mater. Horizons 2017, 4, 1196–1200. [Google Scholar] [CrossRef]
- Celik, S.B.G.; Dominici, S.R.; Filby, B.W.; Das, A.A.K.; Madden, L.A.; Paunov, V.N. Fabrication of human keratinocyte cell clusters for skin graft applications by templating water-in-water Pickering emulsions. Biomimetics 2019, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Takayama, S.; Park, J. Formation and manipulation of cell spheroids using a density adjusted PEG/DEX aqueous two-phase system. Sci. Rep. 2015, 5, 11891. [Google Scholar] [CrossRef]
- Atefi, E.; Lemmo, S.; Fyffe, D.; Luker, G.D.; Tavana, H. High throughput, polymeric aqueous two-phase printing of tumor spheroids. Adv. Funct. Mater. 2014, 24, 6509–6515. [Google Scholar] [CrossRef] [Green Version]
- Atefi, E.; Joshi, R.; Mann, J.A., Jr.; Tavana, H. Interfacial tension effect on cell partition in aqueous two-phase systems. ACS Appl. Mater. Interfaces 2015, 7, 21305–21314. [Google Scholar] [CrossRef]
- Chiew, G.G.Y.; Fu, A.; Perng Low, K.; Qian Luo, K. Physical supports from liver cancer cells are essential for differentiation and remodeling of endothelial cells in a HepG2-HUVEC co-culture model. Sci. Rep. 2015, 5, 10801. [Google Scholar] [CrossRef] [Green Version]
- Lasli, S.; Kim, H.-J.; Lee, K.; Suurmond, C.-A.E.; Goudie, M.; Bandaru, P.; Sun, W.; Zhang, S.; Zhang, N.; Ahadian, S.; et al. A human liver-on-a-chip platform for modeling nonalcoholic fatty liver disease. Adv. Biosyst. 2019, 3, e1900104. [Google Scholar] [CrossRef]
- Timmins, N.E.; Nielsen, L.K. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol. Med. 2007, 140, 141–151. [Google Scholar] [CrossRef]
- Handsley, M.M.; Edwards, D.R. Metalloproteinases and their inhibitors in tumor angiogenesis. Int. J. Cancer 2005, 115, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; González, L.O.; Corte, M.D.; Rodríguez, J.C.; Vázquez, J.; Lamelas, M.L.; Junquera, S.; Merino, A.M.; García-Muñiz, J.L. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br. J. Cancer 2007, 96, 903–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibuya, M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 2001, 26, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, A.; Wu, X.; Wenger, A.; Klagsbrun, M.; Kurschat, P. Endothelial progenitor cell sprouting in spheroid cultures is resistant to inhibition by osteoblasts: A model for bone replacement grafts. FEBS Lett. 2005, 579, 5338–5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfisterer, L.; Korff, T. Spheroid-based in vitro angiogenesis model. Methods Mol. Biol. 2016, 1430, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Haas, T.L.; Davis, S.J.; Madri, J.A. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J. Biol. Chem. 1998, 273, 3604–3610. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Madden, L.A.; Paunov, V.N. Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions. Bioengineering 2022, 9, 126. https://doi.org/10.3390/bioengineering9030126
Wang A, Madden LA, Paunov VN. Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions. Bioengineering. 2022; 9(3):126. https://doi.org/10.3390/bioengineering9030126
Chicago/Turabian StyleWang, Anheng, Leigh A. Madden, and Vesselin N. Paunov. 2022. "Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions" Bioengineering 9, no. 3: 126. https://doi.org/10.3390/bioengineering9030126
APA StyleWang, A., Madden, L. A., & Paunov, V. N. (2022). Vascularized Co-Culture Clusteroids of Primary Endothelial and Hep-G2 Cells Based on Aqueous Two-Phase Pickering Emulsions. Bioengineering, 9(3), 126. https://doi.org/10.3390/bioengineering9030126