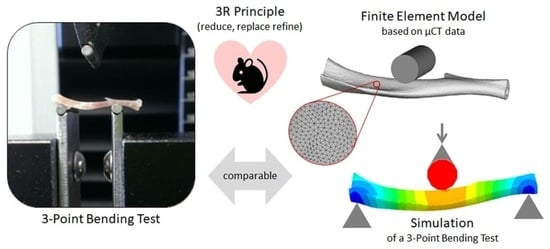
Contribution to the 3R Principle: Description of a Specimen-Specific Finite Element Model Simulating 3-Point-Bending Tests in Mouse Tibiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bone Specimens and µCT (Micro-Computed Tomography) Scanning
2.2. 3-Point Bending Test for FEM Validation
2.3. Establishment of a Finite Element Model to Simulate a 3-Point Bending Setup
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, K.; Alvarez, L.R. An Estimate of the Number of Animals Used for Scientific Purposes Worldwide in 2015. Altern. Lab. Anim. 2019, 47, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Rego Alvarez, L. A summary of EU national statistical reports of animal experiments in 2014–2016. ALTEX 2019, 36, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Franco, N.H. Animal Experiments in Biomedical Research: A Historical Perspective. Animals 2013, 3, 238–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huss, M.K.; Felt, S.A.; Pacharinsak, C. Influence of Pain and Analgesia on Orthopedic and Wound-healing Models in Rats and Mice. Comp. Med. 2019, 69, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, K.J.; Silva, M.J.; Vashishth, D.; Guo, X.E.; van der Meulen, M.C. Establishing biomechanical mechanisms in mouse models: Practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res. 2015, 30, 951–966. [Google Scholar] [CrossRef]
- Oefner, C.; Herrmann, S.; Kebbach, M.; Lange, H.E.; Kluess, D.; Woiczinski, M. Reporting checklist for verification and validation of finite element analysis in orthopedic and trauma biomechanics. Med. Eng. Phys. 2021, 92, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Pistoia, W.; van Rietbergen, B.; Lochmuller, E.M.; Lill, C.A.; Eckstein, F.; Ruegsegger, P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone 2002, 30, 842–848. [Google Scholar] [CrossRef]
- Buckley, J.M.; Loo, K.; Motherway, J. Comparison of quantitative computed tomography-based measures in predicting vertebral compressive strength. Bone 2007, 40, 767–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, A.; Ono, M.; Holmbeck, K.; Li, L.; Kilts, T.M.; Kram, V.; Noonan, M.L.; Yoshioka, Y.; McNerny, E.M.; Tantillo, M.A.; et al. WNT1-induced Secreted Protein-1 (WISP1), a Novel Regulator of Bone Turnover and Wnt Signaling. J. Biol. Chem. 2015, 290, 14004–14018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, M.; Inkson, C.A.; Kilts, T.M.; Young, M.F. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J. Bone Miner. Res. 2011, 26, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reumann, M.K.; Schaefer, J.; Titz, B.; Aspera-Werz, R.H.; Wong, E.T.; Szostak, J.; Haussling, V.; Ehnert, S.; Leroy, P.; Tan, W.T.; et al. E-vapor aerosols do not compromise bone integrity relative to cigarette smoke after 6-month inhalation in an ApoE(-/-) mouse model. Arch. Toxicol. 2020, 94, 2163–2177. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. The effect of porosity and mineral content on the Young’s modulus of elasticity of compact bone. J. Biomech. 1988, 21, 131–139. [Google Scholar] [CrossRef]
- Cheong, V.S.; Roberts, B.C.; Kadirkamanathan, V.; Dall’Ara, E. Positive interactions of mechanical loading and PTH treatments on spatio-temporal bone remodelling. Acta Biomater. 2021, 136, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Arias-Moreno, A.J.; Ito, K.; van Rietbergen, B. Accuracy of beam theory for estimating bone tissue modulus and yield stress from 3-point bending tests on rat femora. J. Biomech. 2020, 101, 109654. [Google Scholar] [CrossRef]
- Bourne, B.C.; van der Meulen, M.C. Finite element models predict cancellous apparent modulus when tissue modulus is scaled from specimen CT-attenuation. J. Biomech. 2004, 37, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, S.; Roberts, M.; Owen, R.; Reilly, G.C.; Bellantuono, I.; Dall’Ara, E. Non-invasive prediction of the mouse tibia mechanical properties from microCT images: Comparison between different finite element models. Biomech. Model. Mechanobiol. 2021, 20, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Baca, V.; Horak, Z.; Mikulenka, P.; Dzupa, V. Comparison of an inhomogeneous orthotropic and isotropic material models used for FE analyses. Med. Eng. Phys. 2008, 30, 924–930. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Nussler, A.K.; Reumann, M.K.; Augat, P.; Menger, M.M.; Ghallab, A.; Hengstler, J.G.; Histing, T.; Ehnert, S. Contribution to the 3R Principle: Description of a Specimen-Specific Finite Element Model Simulating 3-Point-Bending Tests in Mouse Tibiae. Bioengineering 2022, 9, 337. https://doi.org/10.3390/bioengineering9080337
Huang X, Nussler AK, Reumann MK, Augat P, Menger MM, Ghallab A, Hengstler JG, Histing T, Ehnert S. Contribution to the 3R Principle: Description of a Specimen-Specific Finite Element Model Simulating 3-Point-Bending Tests in Mouse Tibiae. Bioengineering. 2022; 9(8):337. https://doi.org/10.3390/bioengineering9080337
Chicago/Turabian StyleHuang, Xiaowei, Andreas K. Nussler, Marie K. Reumann, Peter Augat, Maximilian M. Menger, Ahmed Ghallab, Jan G. Hengstler, Tina Histing, and Sabrina Ehnert. 2022. "Contribution to the 3R Principle: Description of a Specimen-Specific Finite Element Model Simulating 3-Point-Bending Tests in Mouse Tibiae" Bioengineering 9, no. 8: 337. https://doi.org/10.3390/bioengineering9080337
APA StyleHuang, X., Nussler, A. K., Reumann, M. K., Augat, P., Menger, M. M., Ghallab, A., Hengstler, J. G., Histing, T., & Ehnert, S. (2022). Contribution to the 3R Principle: Description of a Specimen-Specific Finite Element Model Simulating 3-Point-Bending Tests in Mouse Tibiae. Bioengineering, 9(8), 337. https://doi.org/10.3390/bioengineering9080337