Ricotta Cheese Whey-Fruit-Based Beverages: Pasteurization Effects on Antioxidant Composition and Color
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
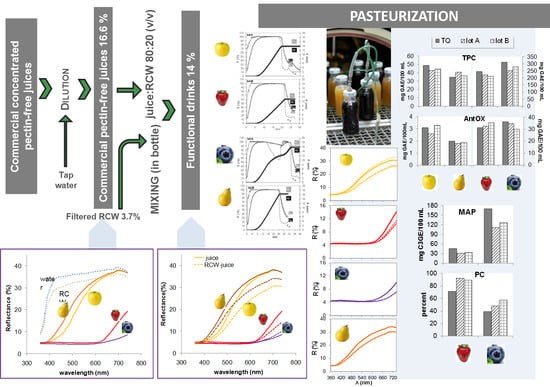
2.2.1. Beverage Preparation
2.2.2. Physicochemical Analysis
2.2.3. Determination of Total Phenolics and Total Anthocyanin Content
2.2.4. Determination of Antioxidant Activity
2.2.5. Color Measurements
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Multifactor Analysis of Variance
3.2. Physicochemical Analysis
3.3. Influence of Blending with RCW on Fruit Juice Color
3.4. Influence of Pasteurization on Fruit-RCW BeveragesColor
3.5. Influence of Fruit Juice Type on Total Phenolics, Total Anthocyanin Content and Antioxidant Activity
3.6. Influence of Pasteurization on Total Phenolics, Total Anthocyanin Content, and Antioxidant Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RCW | Ricotta-cheese whey |
| SSC | Soluble solid content |
| TPC | Total phenolic content |
| MAP | Monomeric anthocyanin pigment |
| AntOx | Antioxidant activity |
| PC | Percent polymeric color |
References
- Hözer, B.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar]
- Shaafsma, G. Lactose and lactose derivatives as bioactive ingredients in human nutrition. Int. Dairy J. 2008, 18, 458–465. [Google Scholar] [CrossRef]
- Manso, M.A.; López-Fandiño, R. κ-Casein macropeptides from cheese whey: physicochemical, biological, nutritional and technological features for possible uses. Food Rev. Int. 2004, 20, 329–355. [Google Scholar] [CrossRef]
- Ko, S.; Kwak, H.-S. Bioactive components in whey products. In Bioactive Components in Milk and Dairy Products; Park, Y.W., Ed.; Wiley-Blackwell: Ames, IA, USA, 2009; pp. 263–284. [Google Scholar]
- Brambilla, A.; Lo Scalzo, R.; Bertolo, G.; Torreggiani, D. Steam-blanched highbush blueberry (Vaccinium corymbosum L.) juice: Phenolic profile and antioxidant capacity in relation to cultivar selection. J. Agric. Food Chem. 2008, 56, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Navindra, P.S. Recent trends and advances in berry health benefits research. J. Agric. Food Chem. 2010, 58, 3869–3870. [Google Scholar]
- Ferreira Wessel, D.; Coelho, E.; Coimbra, A.M. Pear Juice. In Handbook of Functional Beverages and Human Health; Shahidi, F., Alasavar, C., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 475–488. [Google Scholar]
- Hyson, D.H. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Djurić, M.; Carić, M.; Milanović, S.; Tekić, M.; Panić, M. Development of whey-based beverages. Eur. Food Res. Technol. 2004, 219, 321–328. [Google Scholar] [CrossRef]
- Castro, W.F.; Cruz, A.G.; Bisinotto, M.S.; Guerriero, L.M.R.; Faria, J.A.F.; Bolini, H.M.A.; Cunha, R.L.; Deliza, R. Development of probiotic dairy beverages: Rheological properties and application of mathematical models in sensory evaluation. J. Dairy Sci. 2012, 96, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Sakhale, B.K.; Pawar, V.N.; Ranveer, R.C. Studies on the development and storage of whey based RTS beverage from mango cv. Kesar. J. Food Process. Technol. 2012, 3, 148. [Google Scholar]
- Goudarzi, M.; Madadlou, A.; Mousavi, M.E.; Emam-Djomeh, Z. Formulation of apple juice beverages containing whey protein isolate or whey protein hydrolysate based on sensory and physicochemical analysis. Int. J. Dairy Technol. 2015, 68, 70–78. [Google Scholar] [CrossRef]
- Panesar, P.S.; Kennedy, J.F. Biotechnological approaches for the value addition of whey. Crit. Rev. Biotechnol. 2012, 32, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, S.; Curcio, S.; Calabrò, V.; Iorio, G. Bio-ethanol production of ricotta cheese-whey as an effective alternative non-vegetable source. Biomass Bioenerg. 2009, 33, 1687–1697. [Google Scholar] [CrossRef]
- Sansonetti, S.; Curcio, S.; Calabrò, V.; Iorio, G. Optimization of ricotta cheese whey (RCW) fermentation by response surface methodology. Bioresour. Technol. 2010, 101, 9156–9162. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.N.; Vardhanabhuti, B.; Jaramillo, D.P.; Van Zanten, J.N.; Coupland, J.N.; Foegeding, E.A. Stability and mechanism of whey protein soluble aggregates thermally treated with salts. Food Hydrocoll. 2012, 27, 411–420. [Google Scholar] [CrossRef]
- Koffi, E.; Shewfelt, R.; Wicker, L. Storage stability and sensory analysis of UHT-processed whey-banana beverages. J. Food Qual. 2005, 28, 386–401. [Google Scholar] [CrossRef]
- Lee, C.A.; Vickers, Z.M. The astringency of whey protein beverages is caused by their acidity. Int. Dairy J. 2008, 18, 1153–1156. [Google Scholar] [CrossRef]
- LaClair, C.E.; Etzel, R.M. Ingredient and pH are key to clear beverages that contain whey protein. J. Food Sci. 2010, 75, C21–C27. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., An, H., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Sporns, P., Eds.; John Wiley and Sons: New York, NY, USA, 2001; pp. F1.2.1–F1.2.13. [Google Scholar]
- Lo Scalzo, R.; Iannoccari, T.; Summa, C.; Morelli, R.; Rapisarda, P. Effect of thermal treatments on antioxidant and antiradical activity of blood orange juice. Food Chem. 2004, 85, 41–47. [Google Scholar] [CrossRef]
- CIE. Colorimetry—Part 2: CIE Standard Illuminants. [ISO 11664-2:2007]. 2006. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11664:-2:ed-1:v1:en (accessed on 15 March 2015).
- Romano, G.; Baranyai, L.; Gottschalck, K.; Zude, M. An approach for monitoring the moisture content changes of drying banana slices with laser light backscattering imaging. Food Bioprocess Tech. 2008, 4, 410–414. [Google Scholar] [CrossRef]
- CIE. Improvement to Industrial Color Difference Evaluation, CIE Technical Report 142-2001; CIE Central Bureau: Vienna, Austria, 2001.
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Lindbloom, B.J. Color Difference Calculator Version 3.0, 2012. Available online: http://brucelindbloom.com/ColorDifferenceCalc.html (accessed on 19 July 2015).
- Kang, C.W.; Kvam, P.H. Basic Statistical Tools for Improving Quality; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 97–124. [Google Scholar]
- Baccouche, A.; Ennouri, M.; Felfoul, I.; Attia, H. A physical stability study of whey-based prickly-pear beverages. Food Hydrocoll. 2013, 33, 234–244. [Google Scholar] [CrossRef]
- Monti, L.; Povolo, M.; Passolungo, L.; Contarini, G. Chemical characterization of Ricotta-cheese whey (scotta). In Proceedings of the Final Conference of project CANADAIR, Twinning Italy-Canada activities in Research and Innovation in the Agro-Food Area, Lodi, Italy, 15 June 2015; CREA: Roma, Italy, 2015; pp. 60–64. [Google Scholar]
- APAT IRSA-CNR. 2110 Torbidità. In Manual 29:2003 Metodi analitici per le acque; Belli, M., Centiolo, D., de Zorzi, P., Sansone, U., Capri, S., Pagnotta, R., Pettine, M., Eds.; APAT: Roma, Italy, 2003; Volume 1, pp. 177–182. [Google Scholar]
- Ibarz, A.; Pagán, J.; Garza, S. Kinetic models of non-enzymatic browning in apple puree. J. Sci. Food Agric. 2000, 80, 1162–1168. [Google Scholar] [CrossRef]
- Ibarz, A.; Pagán, J.; Garza, S. Kinetic models for colour changes in pear puree during heating at relatively high temperatures. J. Food Eng. 1999, 39, 415–422. [Google Scholar] [CrossRef]
- Ngo, T.; Wrolstad, R.E.; Zhao, Y. Color quality of Oregon strawberries—Impact of genotype, composition, and processing. J. Food Sci. 2007, 72, C25–C32. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Impact of juice processing on blueberry anthocyanins and polyphenolics: Comparison of two pretreatments. J. Food Sci. 2002, 67, 1660–1667. [Google Scholar] [CrossRef]
- Cserhalmi, Z.; Sass-Kiss, Á.; Tóth-Markus, M.; Lechner, N. Study of pulsed electric field treated citrus juices. Innov. Food Sci. Emerg. 2006, 7, 49–54. [Google Scholar] [CrossRef]
- Cortellino, G.; Rizzolo, A.; Cattaneo, T.M.P. Ricotta-cheese whey- fruit based functional drinks. In Proceedings of the Final Conference of project CANADAIR, Twinning Italy-Canada activities in Research and Innovation in the Agro-Food Area, Lodi, Italy, 15 June 2015; CREA: Roma, Italy, 2015; pp. 65–70. [Google Scholar]
- Chen, Y.; Yu, L.J.; Rupasinghe, H.P.V. Effect of thermal and non-thermal pasteurisation on the microbial inactivation and phenolic degradation in fruit juice: A mini-review. J. Sci. Food Agric. 2013, 93, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Noci, F.; Riener, J.; Walkling-Ribeiro, M.; Cronin, D.A.; Morgan, D.J.; Lyng, J.G. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. J. Food Eng. 2008, 85, 141–146. [Google Scholar] [CrossRef]
- Odrizola-Serrano, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239–248. [Google Scholar] [CrossRef]
- Buckow, R.; Kastell, A.; Terefe, N.S.; Versteeg, C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J. Agric. Food Chem. 2010, 58, 10078–10084. [Google Scholar] [CrossRef] [PubMed]
- Kechinski, C.P.; Guimarães, P.V.R.; Noreña, C.P.Z.; Tessaro, I.C.; Marczak, L.D.F. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J. Food Sci. 2010, 75, C173–C176. [Google Scholar] [CrossRef] [PubMed]
- Martynenko, A.; Chen, Y. Degradation kinetics of total anthocyanins and formation of polymeric color in blueberry hydrothermodynamic (HTD) processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Türkyilmaz, M.; Özkan, M. Kinetics of anthocyanin degradation and polymeric colour formation in black carrot juice concentrates during storage. Int. J. Food Sci. Technol. 2012, 47, 2273–2281. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Toledo, R. Antioxidant activity of water-soluble Maillard reaction products. Food Chem. 2005, 93, 273–278. [Google Scholar] [CrossRef]





| Effects | TA | SSC | pH | NTU | TPC | AntOx | MAP | PC | L* | h° | C* | E* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘yellow’ beverages | ||||||||||||
| J | *** | 0.58 | *** | *** | *** | *** | *** | *** | *** | *** | ||
| H | * | * | *** | *** | 0.12 | *** | *** | *** | *** | *** | ||
| J × H | 0.46 | 0.21 | *** | *** | ** | *** | *** | *** | *** | *** | ||
| ‘red’ beverages | ||||||||||||
| J | *** | *** | *** | *** | *** | * | *** | *** | *** | *** | *** | *** |
| H | 0.52 | *** | *** | *** | *** | 0.17 | *** | 0.10 | * | * | ** | *** |
| J × H | 0.14 | *** | *** | *** | *** | *** | *** | ** | *** | ** | 0.23 | *** |
| Effects | ∆TPC | ∆AntOx | ∆MAP | ∆PC | ∆L* | ∆h° | ∆C* | ∆Eab* | ∆E00* |
|---|---|---|---|---|---|---|---|---|---|
| ‘yellow’ beverages | |||||||||
| J | *** | 0.55 | *** | *** | *** | ** | * | ||
| H | 0.52 | *** | *** | *** | 0.12 | *** | *** | ||
| J × H | * | *** | *** | *** | ** | *** | *** | ||
| ‘red’ beverages | |||||||||
| J | *** | *** | *** | 0.07 | ** | *** | *** | *** | *** |
| H | * | 0.27 | * | 0.43 | * | * | ** | ** | ** |
| J × H | *** | *** | * | 0.06 | *** | ** | 0.18 | 0.62 | 0.82 |
| ‘Yellow’ Beverages | |||||||
| Apple | Pear | p-value 1,2 | |||||
| lot A | lot B | p-value 1 | lot A | lot B | p-value 1 | ||
| Titratable acidity (meq/100 mL) | 6.82 ± 0.13 | 6.70 ± 0.15 | NS | 3.48 ± 0.07 | 3.26 ± 0.05 | * | *** |
| SSC (g/100 g) | 14.21 ± 0.16 | 14.37 ± 0.01 | NS | 14.24 ± 0.02 | 14.31 ± 0.01 | ** | NS |
| pH | 3.79 ± 0.0006 | 3.80 ± 0.0006 | NS | 4.27 ± 0.0005 | 4.51 ± 0.0006 | *** | *** |
| Turbidity (NTU) | 50.0 ± 1.0 | 25.3 ± 0.60 | *** | 23.9 ± 0.58 | 24.7 ± 0.58 | * | * |
| ‘Red’ Beverages | |||||||
| Blueberry | Strawberry | p-value 1,2 | |||||
| lot A | lot B | p-value 1 | lot A | lot B | p-value 1 | ||
| Titratable acidity (meq/100 mL) | 18.84 ± 0.35 | 19.13 ± 0.54 | NS | 16.14 ± 0.17 | 15.50 ± 0.22 | NS | *** |
| SSC (g/100 g) | 14.07 ± 0.10 | 13.99 ± 0.01 | NS | 14.74 ± 0.03 | 14.32 ± 0.04 | *** | *** |
| pH | 3.31 ± 0.0006 | 3.38 ± 0.0005 | *** | 3.61 ± 0.0001 | 3.61 ± 0.0002 | NS | *** |
| Turbidity (NTU) | 190.0 ± 1.0 | 188.5 ± 2.5 | NS | 91.7 ± 1.5 | 78.3 ± 1.5 | *** | *** |
| Apple | Pear | Blueberry | Strawberry | ||
|---|---|---|---|---|---|
| L* | juice | 57.98 ± 0.01 | 60.90 ± 0.01 | 24.87 ± 0.01 | 26.90 ± 0.01 |
| RCW-TQ | 51.29 ± 0.29 | 56.68 ± 0.08 | 25.13 ± 0.01a | 25.82 ± 0.01 | |
| p-value 1 | *** | *** | *** | *** | |
| h° | juice | 84.73 ± 0.01 | 90.99 ± 0.04 | 361.35 ± 0.15 | 359.51 ± 0.06 |
| RCW-TQ | 81.76 ± 0.08 | 88.63 ± 0.01 | 354.09 ± 0.33 | 374.73 ± 0.14 | |
| p-value 1 | *** | *** | *** | *** | |
| C* | juice | 35.87±0.01 | 28.25±0.01 | 2.14±0.01 | 3.22±0.01 |
| RCW-TQ | 29.89±0.22 | 26.81±0.06 | 2.41±0.02 | 3.41±0.01 | |
| p-value 1 | *** | *** | *** | *** | |
| E* | juice | 68.18±0.01 | 67.13±0.01 | 24.96±0.01 | 27.09±0.01 |
| RCW-TQ | 59.36±0.35 | 62.70±0.06 | 25.24±0.01 | 26.04±0.01 | |
| p-value 1 | *** | *** | *** | *** |
| Apple | Pear | |||||
| lot A | lot B | p-value 1 | lot A | lot B | p-value 1 | |
| ΔL* | –5.55 ± 0.28 | +1.22 ± 0.35 | *** | –4.28 ± 0.08 | –4.62 ± 0.07 | ** |
| ΔC* | –3.65 ± 0.19 | +0.536 ± 0.10 | *** | –0.90 ± 0.12 | –1.11 ± 0.07 | NS |
| Δh° | –4.16 ± 0.12 | +0.68 ± 0.07 | *** | –3.40 ± 0.02 | –3.53 ± 0.01 | *** |
| ΔEab* | 6.94 ± 0.34 | 1.38 ± 0.35 | *** | 4.64 ± 0.09 | 5.01 ± 0.05 | ** |
| ΔE00* | 6.00 ± 0.30 | 1.27 ± 0.34 | *** | 4.00 ± 0.58 | 4.67 ± 0.06 | NS |
| Blueberry | Strawberry | |||||
| lot A | lot B | p-value 1 | lot A | lot B | p-value 1 | |
| ΔL* | –0.72 ± 0.01 | –0.69 ± 0.02 | NS | –0.58 ± 0.03 | –0.70 ± 0.03 | ** |
| ΔC* | –1.63 ± 0.03 | –1.52 ± 0.05 | * | –1.29 ± 0.02 | –1.10 ± 0.09 | * |
| Δh° | –17.9 ± 2.2 | –10.3 ± 2.6 | * | +1.62 ± 0.26 | +0.17 ± 1.04 | NS |
| ΔEab* | 1.83 ± 0.04 | 1.69 ± 0.06 | * | 1.42 ± 0.01 | 1.30 ± 0.03 | * |
| ΔE00* | 2.34 ± 0.06 | 2.14 ± 0.09 | * | 1.68 ± 0.01 | 1.46 ± 0.11 | * |
| ‘Yellow’ Drinks | Apple | Pear | p-value 1 |
| TPC (mg GAE/100 mL) | 48.7 ± 0.95 | 34.6 ± 0.35 | *** |
| AntOx (mg GAE/100 mL) | 3.07 ± 0.07 | 1.99 ± 0.04 | *** |
| ‘Red’ Drinks | Blueberry | Strawberry | p-value 1 |
| TPC (mg GAE/100 mL) | 310.7 ± 1.9 | 245.2 ± 1.8 | *** |
| MAP (mg C3GE/100 mL) | 170.2 ± 6.0 | 45.8 ± 1.5 | *** |
| AntOx (mg GAE/100 mL) | 36.20 ± 0.96 | 31.33 ± 0.55 | ** |
| Polymeric color (%) | 39.14 ± 0.61 | 71.41 ± 5.33 | *** |
| Blueberry | Strawberry | |||||
|---|---|---|---|---|---|---|
| Past A | Past B | p-value 1 | Past A | Past B | p-value 1 | |
| ΔTPC (mg GAE/100 mL) | –58.8 ± 5.3 | –32.2 ± 5.7 | ** | –23.5 ± 0.5 | –33.1 ± 5.2 | * |
| ΔAntOx (mg GAE/100 mL) | –1.3 ± 0.5 | –5.9 ± 1.2 | ** | +1.2 ± 2.0 | +4.2 ± 0.7 | NS |
| ΔMAP (mg C3GE/100 mL) | –58.6 ± 7.1 | –43.7 ± 4.7 | * | –14.4 ± 1.9 | –12.3 ± 2.2 | NS |
| ΔPC (%) | +8.8 ± 1.8 | +18.2 ± 4.3 | * | +22.1 ± 7.2 | +17.9 ± 6.2 | NS |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzolo, A.; Cortellino, G. Ricotta Cheese Whey-Fruit-Based Beverages: Pasteurization Effects on Antioxidant Composition and Color. Beverages 2017, 3, 15. https://doi.org/10.3390/beverages3010015
Rizzolo A, Cortellino G. Ricotta Cheese Whey-Fruit-Based Beverages: Pasteurization Effects on Antioxidant Composition and Color. Beverages. 2017; 3(1):15. https://doi.org/10.3390/beverages3010015
Chicago/Turabian StyleRizzolo, Anna, and Giovanna Cortellino. 2017. "Ricotta Cheese Whey-Fruit-Based Beverages: Pasteurization Effects on Antioxidant Composition and Color" Beverages 3, no. 1: 15. https://doi.org/10.3390/beverages3010015
APA StyleRizzolo, A., & Cortellino, G. (2017). Ricotta Cheese Whey-Fruit-Based Beverages: Pasteurization Effects on Antioxidant Composition and Color. Beverages, 3(1), 15. https://doi.org/10.3390/beverages3010015