Process Parameters Affecting the Synthesis of Natural Flavors by Shiitake (Lentinula edodes) during the Production of a Non-Alcoholic Beverage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Media
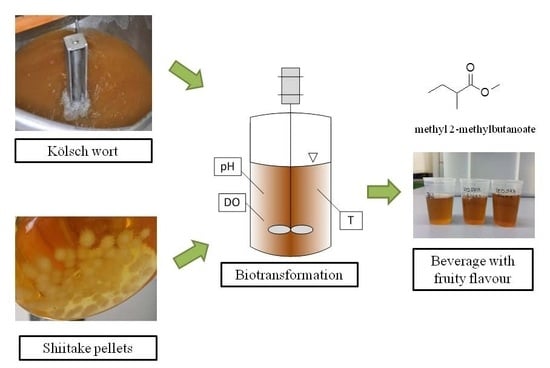
2.2. Wort Production
2.3. Pre-Culture Cultivation
2.4. Biotransformation
2.5. Measurement of Cell Dry Weight
2.6. Characterization of Fungal Morphology
2.7. Flavor Analysis
2.8. Measurement of the Oxygen Gradient
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microprofiling
3.2. Effect of Volumetric Power Input and Maximum Shear Rate
3.2.1. Morphology and Biomass Growth
3.2.2. Flavor Production
3.3. Effect of Inoculum Concentration
3.3.1. Morphology and Biomass Growth
3.3.2. Flavor Production
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [PubMed]
- Hatvani, N.; Mécs, I. Production of laccase and manganese peroxidase by Lentinus edodes on malt-containing by-product of the brewing process. Process Biochem. 2001, 37, 491–496. [Google Scholar] [CrossRef]
- Abraham, B.G.; Berger, R.G. Higher Fungi for Generating Aroma Components through Novel Biotechnologies. J. Agric. Food Chem. 1994, 42, 2344–2348. [Google Scholar] [CrossRef]
- Zhang, Y.; Fraatz, M.A.; Horlamus, F.; Quitmann, H.; Zorn, H. Identification of Potent Odorants in a Novel Nonalcoholic Beverage Produced by Fermentation of Wort with Shiitake (Lentinula edodes). J. Agric. Food Chem. 2014, 62, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hartung, N.M.; Fraatz, M.A.; Zorn, H. Quantification of key odor-active compounds of a novel nonalcoholic beverage produced by fermentation of wort by shiitake (Lentinula edodes) and aroma genesis studies. Food Res. Int. 2015, 70, 23–30. [Google Scholar] [CrossRef]
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged Culture Fermentation of “Higher Fungi”: The Macrofungi. Adv. Appl. Microbiol. 2008, 63, 33–103. [Google Scholar] [PubMed]
- Tang, Y.J.; Zu, L.W.; Li, H.M.; Li, D.S. Submerged Culture of Mushrooms in Bioreactors—Challenges, Current State-of-the-Art, and Future Prospects. Food Technol. Biotechnol. 2007, 45, 221–229. [Google Scholar]
- Druzinec, D.; Salzig, D.; Kraume, M.; Czermak, P. Micro-bubble aeration in turbulent stirred bioreactors: Coalescence behavior in Pluronic F68 containing cell culture media. Chem. Eng. Sci. 2015, 126, 160–168. [Google Scholar] [CrossRef]
- Perpète, P.; Collin, S. Contribution of 3-Methylthiopropionaldehyde to the Worty Flavor of Alcohol-Free Beers. J. Agric. Food Chem. 1999, 47, 2374–2378. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.; Ulbrecht, J.J. Measurement of shear rate on an agitator in a fermentation broth. In Biotechnology Progress: Scale-Up and Mixing; Ho, C.S., Oldshue, J.Y., Eds.; American Institute of Chemical Engineers: New York, NY, USA, 1987; pp. 72–81. [Google Scholar]
- Schügerl, K.; Wittler, R.; Lorenz, T. The use of molds in pellet form. Trends Biotechnol. 1983, 1, 120–123. [Google Scholar] [CrossRef]
- Prosser, J.I.; Tough, A.J. Growth Mechanisms and Growth Kinetics of Filamentous Microorganisms. Crit. Rev. Biotechnol. 2008, 10, 253–274. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of Filamentous Fungi in Submerged Culture: Problems and Possible Solutions. Crit. Rev. Biotechnol. 2008, 20, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.C.; Grulke, E.A.; Reddy, C.A. Determination of the respiration kinetics for mycelial pellets of Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1992, 58, 1740–1745. [Google Scholar] [PubMed]
- Ha, H.C.; Honda, Y.; Watanabe, T.; Kuwahara, M. Production of manganese peroxidase by pellet culture of the lignin-degrading basidiomycete, Pleurotus ostreatus. Appl. Microbiol. Biotechnol. 2001, 55, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Leisola, M.S.; Fiechter, A. Ligninase production in agitated conditions by Phanerochaete chrysosporium. FEMS Microbiol. Lett. 1985, 29, 33–36. [Google Scholar] [CrossRef]
- Tepwong, P.; Giri, A.; Ohshima, T. Effect of mycelial morphology on ergothioneine production during liquid fermentation of Lentinula edodes. Mycoscience 2012, 53, 102–112. [Google Scholar] [CrossRef]
- Gehrig, I.; Bart, H.J.; Anke, T.; Germerdonk, R. Influence of morphology and rheology on the production characteristics of the basidiomycete Cyathus striatus. Biotechnol. Bioeng. 1998, 59, 525–533. [Google Scholar] [CrossRef]
- Fang, Q.H.; Tang, Y.J.; Zhong, J.J. Significance of inoculation density control in production of polysaccharide and ganoderic acid by submerged culture of Ganoderma lucidum. Process Biochem. 2002, 37, 1375–1379. [Google Scholar] [CrossRef]
- Park, J.P.; Kim, Y.M.; Kim, S.W.; Hwang, H.J.; Cho, Y.J.; Lee, Y.S.; Song, C.H.; Yun, J.W. Effect of agitation intensity on the exo-biopolymer production and mycelial morphology in Cordyceps militaris. Lett. Appl. Microbiol. 2002, 34, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Babič, J.; Pavko, A. Enhanced enzyme production with the pelleted form of D. squalens in laboratory bioreactors using added natural lignin inducer. J. Ind. Microbiol. Biotechnol. 2012, 39, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, H.J.; Xu, C.P. Culture characterization of exopolysaccharides with antioxidant activity produced by Pycnoporus sanguineus in stirred-tank and airlift reactors. J. Taiwan Inst. Chem. Eng. 2014, 45, 2075–2080. [Google Scholar] [CrossRef]
- Tinoco-Valencia, R.; Gómez-Cruz, C.; Galindo, E.; Serrano-Carreón, L. Toward an understanding of the effects of agitation and aeration on growth and laccases production by Pleurotus ostreatus. J. Biotechnol. 2014, 177, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rocha, F.J.; Guillén-Navarro, K.; Sánchez, J.E.; Vázquez-Duhalt, R. Growth characteristics of Pleurotus ostreatus in bioreactors. Biotechnol. Tech. 1999, 13, 29–32. [Google Scholar] [CrossRef]
- Enman, J.; Hodge, D.; Berglund, K.A.; Rova, U. Production of the Bioactive Compound Eritadenine by Submerged Cultivation of Shiitake (Lentinus edodes) Mycelia. J. Agric. Food Chem. 2008, 56, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Lomascolo, A.; Stentelaire, C.; Asther, M.; Lesage-Meessen, M. Basidiomycetes as new biotechnological tools to generate natural aromatic flavors for the food industry. Trends Biotechnol. 1999, 17, 282–289. [Google Scholar] [CrossRef]
- Mishra, C.; Leatham, G.F. Recovery and fractionation of the extracellular degradative enzymes from Lentinula edodes cultures cultivated on a solid lignocellulosic substrate. J. Ferment. Bioeng. 1990, 69, 8–15. [Google Scholar] [CrossRef]
- Lee, J.S.; Jung, W.C.; Park, S.J.; Lee, K.E.; Shin, W.C.; Hong, E.K. Culture conditions and medium components for the production of mycelial biomass and exo-polysaccharides with Paecilomyces japonica in liquid culture. J. Biosci. Bioeng. 2013, 115, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lee, J.S.; Cho, J.Y.; Kim, Y.E.; Hong, E.K. Process development for mycelial growth and polysaccharide production in Tricholoma matsutake liquid culture. J. Biosci. Bioeng. 2010, 109, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Bae, J.T.; Pyo, H.B.; Choe, T.B.; Kim, S.W.; Hwang, H.J.; Yun, J.W. Submerged culture conditions for the production of mycelial biomass and exopolysaccharides by the edible Basidiomycete Grifola frondosa. Enzyme Microb. Technol. 2004, 35, 369–376. [Google Scholar] [CrossRef]
- Özel, Y.K.; Gedikli, S.; Aytar, P.; Ünal, A.; Yamaç, M.; Çabuk, A.; Kolankaya, N. New fungal biomasses for cyanide biodegradation. J. Biosci. Bioeng. 2010, 110, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.N.; Wang, T.C.; Lin, T.C.; Guo, J.H. Optimal conditions for mycelia biomass and extracellular polysaccharides of Grifola frondosa: Effect of agitation speed, inoculum ratio and initial pH. Afr. J. Biotechnol. 2012, 11. [Google Scholar] [CrossRef]
- Berovič, M.; Habijanič, J.; Zore, I.; Wraber, B.; Hodžar, D.; Boh, B.; Pohleven, F. Submerged cultivation of Ganoderma lucidum biomass and immunostimulatory effects of fungal polysaccharides. J. Biotechnol. 2003, 103, 77–86. [Google Scholar] [CrossRef]
- Gong, H.G.; Jian-Jiang, Z. Hydrodynamic Shear Stress Affects Cell Growth and Metabolite Production by Medicinal Mushroom Ganoderma lucidum. Chin. J. Chem. Eng. 2005, 13, 426–428. [Google Scholar]
- Fenice, M.; Giovannozzi Sermanni, G.; Federici, F.; Dannibale, A. Submerged and solid-state production of laccase and Mn-peroxidase by Panus tigrinus on olive mill wastewater-based media. J. Biotechnol. 2003, 100, 77–85. [Google Scholar] [CrossRef]
- D’Annibale, A.; Quaratino, D.; Federici, F.; Fenice, M. Effect of agitation and aeration on the reduction of pollutant load of olive mill wastewater by the white-rot fungus Panus tigrinus. Biochem. Eng. J. 2006, 29, 243–249. [Google Scholar] [CrossRef]
- Cho, E.J.; Oh, J.Y.; Chang, H.Y.; Yun, J.W. Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J. Biotechnol. 2006, 127, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, P.; Takenberg, M.; Hartwig, S.; Beutel, S.; Berger, R.G.; Scheper, T. Cultivation of shear stress sensitive microorganisms in disposable bag reactor systems. J. Biotechnol. 2013, 167, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.G.; Olías, R.; Luaces, P.; Sanz, C. Biosynthesis of Strawberry Aroma Compounds through Amino Acid Metabolism. J. Agric. Food Chem. 2002, 50, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Rowan, D.D.; Lane, H.P.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of 2-Methylbutyl, 2-Methyl-2-butenyl, and 2-Methylbutanoate Esters in Red Delicious and Granny Smith Apples Using Deuterium-Labeled Substrates. J. Agric. Food Chem. 1996, 44, 3276–3285. [Google Scholar] [CrossRef]
- Matich, A.; Rowan, D. Pathway Analysis of Branched-Chain Ester Biosynthesis in Apple Using Deuterium Labeling and Enantioselective Gas Chromatography-Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 2727–2735. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.C.; Liau, C.B. Effects of cultivating conditions on the mycelial growth of Ganoderma lucidum in submerged flask cultures. Bioprocess Eng. 1998, 19, 233–236. [Google Scholar] [CrossRef]
- Lin, J.; Yang, S. Mycelium and polysaccharide production of Agaricus blazei Murrill by submerged fermentation. J. Microbiol. Immunol. Infect. 2006, 39, 98–108. [Google Scholar] [PubMed]
- Prasad, K.K.; Mohan, S.V.; Bhaskar, Y.V.; Ramanaiah, S.V.; Babu, V.L.; Pati, B.R.; Sarma, P.N. Laccase production using Pleurotus ostreatus 1804 immobilized on PUF cubes in batch and packed bed reactors: Influence of culture conditions. J. Microbiol. 2005, 43, 301–307. [Google Scholar] [PubMed]
- Prasad, K.K.; Mohan, S.V.; Rao, R.S.; Pati, B.R.; Sarma, P. Laccase production by Pleurotus ostreatus 1804: Optimization of submerged culture conditions by Taguchi DOE methodology. Biochem. Eng. J. 2005, 24, 17–26. [Google Scholar] [CrossRef]
- Yahaya, Y.A.; Don, M.M.; Yahaya, A.S. The effect of culture conditions on the growth of T. lactinea and anti-inflammatory activities via in vitro inhibition of hyaluronidase and lipoxygenase enzyme activities. J. Taiwan Inst. Chem. Eng. 2014, 45, 2054–2059. [Google Scholar]
- Qinnghe, C.; Xiaoyu, Y.; Tiangui, N.; Cheng, J.; Qiugang, M. The screening of culture condition and properties of xylanase by white-rot fungus Pleurotus ostreatus. Process Biochem. 2004, 39, 1561–1566. [Google Scholar] [CrossRef]







| Agitation Rate (rpm) | Max. Shear Rate (1/s) | P/V 1 (W/m3) | Xstart (mg/L) | Xfinal 2 (mg/L) | Biomass Productivity (mg/L h) |
|---|---|---|---|---|---|
| 100 | 247 | 1.7 | 35 | 112 | 1.08 |
| 150 | 453 | 5.7 | 35 | 167 | 1.84 |
| 250 | 974 | 26.2 | 35 | 231 | 2.72 |
| Inoculum Volume (mL) | Initial BDW 1 (mg/L) | Xfinal 2 (mg/L) | Biomass Productivity (mg/L h) |
|---|---|---|---|
| 40 | 21 | 175 | 2.14 |
| 80 | 35 | 225 | 2.63 |
| 160 | 68 | 205 | 1.90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özdemir, S.; Heerd, D.; Quitmann, H.; Zhang, Y.; Fraatz, M.A.; Zorn, H.; Czermak, P. Process Parameters Affecting the Synthesis of Natural Flavors by Shiitake (Lentinula edodes) during the Production of a Non-Alcoholic Beverage. Beverages 2017, 3, 20. https://doi.org/10.3390/beverages3020020
Özdemir S, Heerd D, Quitmann H, Zhang Y, Fraatz MA, Zorn H, Czermak P. Process Parameters Affecting the Synthesis of Natural Flavors by Shiitake (Lentinula edodes) during the Production of a Non-Alcoholic Beverage. Beverages. 2017; 3(2):20. https://doi.org/10.3390/beverages3020020
Chicago/Turabian StyleÖzdemir, Sibel, Doreen Heerd, Hendrich Quitmann, Yanyan Zhang, Marco Alexander Fraatz, Holger Zorn, and Peter Czermak. 2017. "Process Parameters Affecting the Synthesis of Natural Flavors by Shiitake (Lentinula edodes) during the Production of a Non-Alcoholic Beverage" Beverages 3, no. 2: 20. https://doi.org/10.3390/beverages3020020
APA StyleÖzdemir, S., Heerd, D., Quitmann, H., Zhang, Y., Fraatz, M. A., Zorn, H., & Czermak, P. (2017). Process Parameters Affecting the Synthesis of Natural Flavors by Shiitake (Lentinula edodes) during the Production of a Non-Alcoholic Beverage. Beverages, 3(2), 20. https://doi.org/10.3390/beverages3020020