Antioxidant Activity of Commercial Soluble Coffees
Abstract
:1. Introduction
2. Materials and Methods
2.1. Commercial Coffee Samples
2.2. Chemicals and Equipment
2.3. Antioxidant Activity Assessments
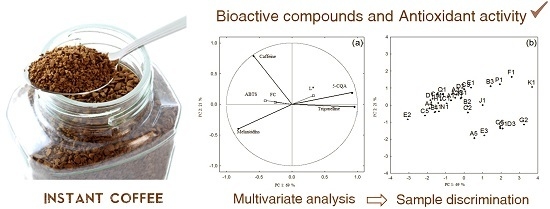
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Coffee Organization (ICO). Trade Statistics Tables. Available online: http://www.ico.org/trade_statistics.asp (accessed on 21 February 2017).
- Durak, A.; Gawlik-Dziki, U. The study of interactions between active compounds of coffee and willow (salix sp.) bark water extract. Biomed. Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, S.P.; Anandharamakrishnan, C. Spray-Freeze-Drying approach for soluble coffee processing and its effect on quality characteristics. J. Food Eng. 2015, 149, 171–180. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Scholz, M.B.S.; Benassi, M.T. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Res. Int. 2014, 61, 61–66. [Google Scholar] [CrossRef]
- Liang, N.; Xue, W.; Kennepohl, P.; Kitts, D.D. Interactions between major chlorogenic acid isomers and chemical changes in coffee brew that affect antioxidant activities. Food Chem. 2016, 213, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.L.B.; Kalschne, D.L.; Ferrão, M.A.G.; Fonseca, A.F.A.; Ferrão, R.G.; Benassi, M.T. Diterpenes in Coffea canephora. J. Food Compos. Anal. 2016, 52, 52–57. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Benassi, M.T. Discrimination between arabica and robusta coffees using hydrosoluble compounds: Is the efficiency of the parameters dependent on the roast degree? Beverages 2015, 1, 127–139. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Viegas, M.C.; Bassoli, D.G.; Benassi, M.T. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 2014, 61, 279–285. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition, and application of coffee and its industrial residues. Food Bioprocess Technol. 2011, 4, 661–672. [Google Scholar] [CrossRef]
- George, S.E.; Ramalakshmi, K.; Rao, L.J.M. A perception on health benefits of coffee. Crit. Rev. Food Sci. Nutr. 2008, 48, 464–486. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.M.; Steluti, J.; Fisberg, R.M.; Marchioni, D.M. Association between coffee consumption and its polyphenols with cardiovascular risk factors: A population-based study. Nutrients 2017, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Freedman, N.D.; Park, Y.; Abnet, C.C.; Hollenbeck, A.R.; Sinha, R. Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 2012, 366, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Yenisetti, S.C.; Muralidhara, M. Beneficial role of coffee and caffeine in neurodegenerative diseases: A minireview. AIMS Public Health 2016, 3, 407–422. [Google Scholar] [CrossRef]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willett, W.; van Dam, R.M.; Hu, F.B. Association of coffee consumption with total and cause-specific mortality in three large prospective cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.B.; Benassi, M.T. Atividade antioxidante e estimativa do teor de melanoidinas em cafés torrados comerciais. Semin. Cienc. Agrar. 2011, 32, 1893–1900. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Stanisz, E.; De Peña, M.P. Relationship between antioxidant capacity, chlorogenic acids and elemental composition of green coffee. LWT—Food Sci. Technol. 2016, 73, 243–250. [Google Scholar] [CrossRef]
- Stelmach, E.; Pohl, P.; Szymczycha-Madeja, A. The content of Ca, Cu, Fe, Mg and Mn and antioxidant activity of green coffee brews. Food Chem. 2015, 182, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Bode, A.M.; Dong, Z. Molecular targets of coffee phytochemicals caffeic acid and chlorogenic acid in chemoprevention. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press-Elsevier: London, UK, 2015; Volume 1, pp. 673–682. [Google Scholar]
- Salomone, F.; Galvano, F.; Volti, G.L. Molecular bases underlying the hepatoprotective effects of coffee. Nutrients 2017, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Wey, F.; Tanokura, M. Chemical changes in the components of coffee beans during roasting. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press-Elsevier: London, UK, 2015; Volume 1, pp. 83–92. [Google Scholar]
- Cruz, D.; Barroso, M.F.; Alves, R.C.; González-García, M.B.; Ramalhosa, M.J.; Duarte, A.J.; Oliveira, M.B.P.P.; Delerue-Matos, C. Assay of Total Antioxidant Capacity of Coffee: Use of a DNA-Based Biosensor. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Academic Press-Elsevier: London, UK, 2015; Volume 1, pp. 963–970. [Google Scholar]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Antioxidant activity, polyphenols, caffeine and melanoidins in soluble coffee: The influence of processing conditions and raw material. Food Chem. 2011, 124, 863–868. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Bassoli, D.G.; Benassi, M.T. Atividade antioxidante de cafés torrado e solúvel: padronização e validação de métodos. Coffee Sci. 2012, 7, 68–75. Available online: http://www.coffeescience.ufla.br/index.php/Coffeescience/article/view/224/pdf (accessed on 20 June 2017).
- Brezová, V.; Šlebodova, A.; Staško, A. Coffee as a source of antioxidants: An EPR study. Food Chem. 2009, 114, 859–868. [Google Scholar] [CrossRef]
- Rodrigues, N.P.; Benassi, M.T.; Bragagnolo, N. Scavenging capacity of coffee brews against oxygen and nitrogen reactive species and the correlation with bioactive compounds by multivariate analysis. Food Res. Int. 2014, 61, 228–235. [Google Scholar] [CrossRef]
- Corso, M.P.; Vignoli, J.A.; Benassi, M.T. Development of an instant coffee enriched with chlorogenic acids. J. Food Sci. Technol. 2016, 53, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, C.T.; Almeida, M.B.; Nixdorf, S.L.; Benassi, M.T. Teores de trigonelina, ácido 5-cafeoilquínico, cafeína e melanoidinas em cafés solúveis comerciais brasileiros. Quim. Nova 2013, 36, 544–548. [Google Scholar] [CrossRef]
- Franca, A.S. Coffee: Decaffeination. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: Oxford, UK, 2015; Volume 2, pp. 232–236. [Google Scholar]
- Daglia, M.; Racchi, M.; Papetti, A.; Lanni, C.; Govoni, S.; Gazzani, G. In vitro and ex vivo antihydroxyl radical activity of green and roasted coffee. J. Agric. Food. Chem. 2004, 52, 1700–1704. [Google Scholar] [CrossRef] [PubMed]

| Parameter 1 | Regular | Decaffeinated | |
|---|---|---|---|
| Traditional | Gourmet | ||
| L* | 19.52–33.0 | 34.63–43.70 | 21.65–26.88 |
| Trigonelline 1 | 0.47–1.60 | 1.15–2.15 | 1.10–1.85 |
| 5-CQA 1 | 0.38–2.66 | 1.15–2.37 | 1.18–2.43 |
| Caffeine 1 | 2.32–4.08 | 2.62–2.85 | 0.06–0.24 |
| Melanoidins 2 | 0.26–0.48 | 0.25–0.39 | 0.31–0.37 |
| Sample 1 | Type 2 | Drying Process | F-C 3 | ABTS 3 |
|---|---|---|---|---|
| A1 * | R | Agglomerated | 13.58 c,d,e (3.93) | 28.42 a,c,d,e,f,g,h,i (18.10) |
| A2 * | R | Agglomerated | 13.83 b,c,d,e (1.07) | 26.20 f,g,h,i,j (17.61) |
| A3 * | R | Agglomerated | 13.94 b,c,d (4.01) | 28.41 a,c,d,e,f,g,h,i (2.67) |
| A4 * | R | Powder | 13.93 b,c,d (1.88) | 30.50 a,b,c,d,e,f,g,h (19.10) |
| A5 ** | D | Agglomerated | 14.19 a,b,c,d,e,f (1.40) | 27.20 c,d,e,f,g,h,i,j (32.33) |
| B1 * | R | Agglomerated | 14.04 b,c (1.83) | 28.45 a,c,d,e,f,g,h,i (14.98) |
| B2 * | G | Freeze-dried | 12.79 e,f (5.37) | 22.48 i,j (7.79) |
| B3 * | R | Powder | 14.40 a,b,c (4.20) | 26.73 e,f,g,h,i,j (12.91) |
| C1 * | R | Agglomerated | 14.41 a,b,c (2.57) | 27.67 d,e,f,g,h,i (14.29) |
| C2 * | G | Freeze-dried | 12.47 f (3.52) | 24.34 h,i,j (10.01) |
| C3 * | R | Powder | 14.23 b,c (5.11) | 29.03 a,c,d,e,f,g,h,i (21.06) |
| C4 * | R | Agglomerated | 13.55 c,d,e,f (3.41) | 31.76 a,b,c,d,e,f,g (8.41) |
| C5 * | R | Agglomerated | 14.16 b,c (3.98) | 31.69 a,b,c,d,e,f,g (19.95) |
| C6 * | D | Agglomerated | 14.06 b,c (1.81) | 25.17 g,h,i,j (9.31) |
| D1 * | R | Agglomerated | 14.77 a,b (0.93) | 32.26 a,b,c,d,e,f (14.15) |
| D2 * | R | Powder | 15.41 a (5.31) | 32.14 a,b,c,d,e,f (9.23) |
| D3 * | D | Agglomerated | 14.27 b,c (1.90) | 29.30 a,b,c,d,e,f,g,h,i (23.39) |
| E1 * | R | Agglomerated | 14.60 a,b,c (5.21) | 29.14 a,b,c,d,e,f,g,h,i (1.87) |
| E2 *** | R | Powder | 15.22 a,b,c (5.26) | 36.65 a,b,c,d,e,f,g (10.49) |
| E3 * | D | Agglomerated | 14.41 a,b,c (2.27) | 34.92 a,b,c (1.01) |
| F1 * | R | Agglomerated | 14.88 a,b (5.97) | 33.51 a,b,c,d,e (7.42) |
| G1 * | R | Powder | 14.39 a,b,c (2.81) | 34.91 a,b,c (19.11) |
| G2 *** | D | Powder | 13.64 a,b,c,d,e,f (0.78) | 31.31 a,b,c,d,e,f,g,h,i,j (6.44) |
| H1 * | R | Powder | 14.24 b,c (2.67) | 33.16 a,b,c,d,e (0.26) |
| I1 * | R | Agglomerated | 14.64 a,b,c (3.04) | 34.32 a,b,c,d (12.10) |
| J1 * | R | Agglomerated | 9.91 g (15.62) | 20.39 j (31.88) |
| K1 * | G | Freeze-dried | 12.83 d,e,f (4.92) | 27.05 e,f,g,h,i,j (6.69) |
| L1 *** | R | Agglomerated | 14.55 a,b,c,d,e (4.53) | 32.95 a,b,c,d,e,f,g,h,i (2.98) |
| M1 ** | R | Agglomerated | 14.78 a,b,c (0.29) | 36.69 a,b (3.05) |
| N1 *** | R | Agglomerated | 13.87 a,b,c,d,e,f (4.37) | 37.02 a,b,c,d,e,f,g (3.88) |
| O1 *** | D | Agglomerated | 14.40 a,b,c,d,e (2.65) | 25.72 a,b,c,d,e,f,g,h,i,j (3.68) |
| P1 *** | R | Agglomerated | 13.72 a,b,c,d,e,f (2.49) | 29.11 a,b,c,d,e,f,g,h,i,j (6.83) |
| Q1 * | R | Agglomerated | 14.32 a,b,c (4.90) | 36.06 b (2.60) |
| Average | 14.01 | 30.14 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcucci, C.T.; Dias, R.C.E.; Almeida, M.B.; Benassi, M.D.T. Antioxidant Activity of Commercial Soluble Coffees. Beverages 2017, 3, 27. https://doi.org/10.3390/beverages3020027
Marcucci CT, Dias RCE, Almeida MB, Benassi MDT. Antioxidant Activity of Commercial Soluble Coffees. Beverages. 2017; 3(2):27. https://doi.org/10.3390/beverages3020027
Chicago/Turabian StyleMarcucci, Carolina Tolentino, Rafael Carlos Eloy Dias, Mariana Bortholazzi Almeida, and Marta De Toledo Benassi. 2017. "Antioxidant Activity of Commercial Soluble Coffees" Beverages 3, no. 2: 27. https://doi.org/10.3390/beverages3020027
APA StyleMarcucci, C. T., Dias, R. C. E., Almeida, M. B., & Benassi, M. D. T. (2017). Antioxidant Activity of Commercial Soluble Coffees. Beverages, 3(2), 27. https://doi.org/10.3390/beverages3020027