Evaluation of Macerating Pectinase Enzyme Activity under Various Temperature, pH and Ethanol Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polygalacturonase
2.1.1. Assay Method and Treatments
2.1.2. Standard Curve
2.1.3. Samples
2.2. Hemicellulase (Mannanase)
2.2.1. Assay Method and Treatments
2.2.2. Standard Curve
2.2.3. Samples
2.3. Protease
3. Results
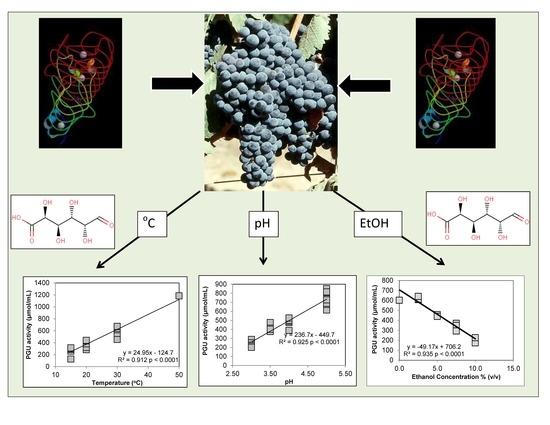
3.1. Polygalacturonase
3.2. Hemicellulase (Mannanase)
3.3. Protease
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krug, K. Problems in the production of apple juice and apple juice concentrates. Flüss. Obst. 1969, 36, 277–283, 333–343. [Google Scholar]
- Meyer, A.S.; Köser, C.; Adler-Nissen, J. Efficiency of enzymatic and other alternative clarification and fining treatments on turbidity and haze in cherry juice. J. Agric. Food Chem. 2001, 49, 3644–3650. [Google Scholar] [CrossRef] [PubMed]
- Landbo, A.-K.R.; Pinelo, M.; Vikbjerg, A.F.; Let, M.B.; Meyer, A.S. Protease-assisted clarification of black currant juice: Synergy with other clarifying agents and effects on the phenol content. J. Agric. Food Chem. 2006, 54, 6554–6563. [Google Scholar] [CrossRef] [PubMed]
- Dawes, H.; Boyes, S.; Keene, J.; Heatherbell, D. Protein instability of wines—Influence of protein isolelectric point. Am. J. Enol. Vitic. 1994, 45, 319–326. [Google Scholar]
- Dawes, H.; Struebi, P.; Keene, J. Kiwifruit juice clarification using a fungal Proteolytic enzyme. J. Food Sci. 1994, 59, 858–861. [Google Scholar] [CrossRef]
- Jiang, J.; Paterson, A.; Piggott, J.R. Effects of Pectolytic enzyme treatments on Anthocyanins in raspberry juice. Int. J. Food Sci. Technol. 1990, 25, 596–600. [Google Scholar] [CrossRef]
- Van Rensburg, P.; Pretorius, I.S. Enzymes in winemaking: Harnessing natural catalysts for efficient biotransformation—A review. S. Afr. J. Enol. Vitic. 2000, 21, 52–73. [Google Scholar]
- Ough, C.S.; Berg, H.W. The effect of two commercial Pectic enzymes on grape musts and wines. Am. J. Enol. Vitic. 1974, 25, 208–211. [Google Scholar]
- Ough, C.S.; Noble, A.C.; Temple, D. Pectic enzyme effects on red grapes. Am. J. Enol. Vitic. 1975, 26, 195–200. [Google Scholar]
- Di Profio, F.D.; Reynolds, A.G.; Kasimos, A. Canopy management and enzyme impacts on Merlot, Cabernet franc, and Cabernet Sauvignon. II. Wine composition and quality. Am. J. Enol. Vitic. 2011, 62, 152–168. [Google Scholar] [CrossRef]
- Kashyap, D.R.; Vohra, P.K.; Chopra, S.; Tewari, R. Applications of pectinases in the commercial sector: A review. Bioresour. Technol. 2001, 77, 215–227. [Google Scholar] [CrossRef]
- King, M.R.; White, B.A.; Blaschek, H.P.; Chassy, B.M.; Mackie, R.I.; Cann, I.K.O. Purification and characterization of a thermostable α-galactosidase from Thermoanaerobacterium polysaccharolyticum. J. Agric. Food Chem. 2002, 50, 5676–5682. [Google Scholar] [CrossRef] [PubMed]
- Pilnik, W.; Rombouts, F.M. Pectic enzymes. In Enzymes and Food Processing; Birch, G., Blakebrough, N., Parker, K.J., Eds.; Applied Science Publishing: London, UK, 1981; pp. 105–128. [Google Scholar]
- Sharma, N.; Rathore, M.; Sharma, M. Microbial pectinase: Sources, characterization and applications. Rev. Environ. Sci. Biotechnol. 2013, 12, 45–60. [Google Scholar] [CrossRef]
- Förster, H. Pectinesterases from Phytophthora infestans. Methods Enzymol. 1988, 161, 355–361. [Google Scholar]
- Nakagawa, T.; Miyaji, T.; Yurimoto, H.; Sakai, Y.; Kato, N.; Tomizuka, N. A methylotrophic pathway participates in pectin utilization by Candida boidinii. Appl. Environ. Microbiol. 2000, 66, 4253–4257. [Google Scholar] [CrossRef] [PubMed]
- Demir, N.; Acar, J.; Sarioğlu, K.; Mutlu, M. The use of commercial pectinase in fruit juice industry. Part 3: Immobilized pectinase for mash treatment. J. Food Eng. 2001, 47, 471–474. [Google Scholar] [CrossRef]
- Grassin, C.; Fauquembergue, P. Application of pectinases in beverages. Prog. Biotechnol. 1996, 14, 453–462. [Google Scholar]
- Zhang, H.; Edward, E.; Woodams, E.E.; Hang, Y.D. Influence of pectinase treatment on fruit spirits from apple mash, juice and pomace. Process Biochem. 2011, 46, 1909–1913. [Google Scholar] [CrossRef]
- Croak, S.; Corredig, M. The role of pectin in orange juice stabilization: Effect of pectin methylesterase and pectinase activity on the size of cloud particles. Food Hydrocoll. 2006, 20, 961–965. [Google Scholar] [CrossRef]
- Willems, J.L.; Low, N.H. Oligosaccharide formation during commercial pear juice processing. Food Chem. 2016, 204, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Bellworthy, S.J.; Reader, H.P.; Watkins, S.J. Effect of enzymes during vinification on color and sensory properties of port wines. Am. J. Enol. Vitic. 1999, 50, 271–276. [Google Scholar]
- Tapre, A.R.; Jain, R.K. Pectinases: Enzymes for fruit processing industry. Int. Food Res. J. 2014, 21, 447–453. [Google Scholar]
- Ghose, T.K.; Bisaria, V.S. Measurement of Hemicellulase activities. Part 1: Xylanases. Pure Appl. Chem. 1987, 59, 1739–1752. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Ann. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.K. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- Parekh, V.J.; Rathod, V.K.; Pandit, A.B. Substrate hydrolysis: Methods, mechanism, and industrial applications of substrate hydrolysis. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 2, pp. 103–118. [Google Scholar]
- Rizzo, V.; Tomaselli, F.; Gentile, A.; la Malfa, S.; Maccarone, E. Rheological properties and sugar composition of locust bean gum from different carob varieties (Ceratonia siliqua L.). J. Agric. Food Chem. 2004, 52, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Smaali, I.; Rémond, C.; Skhiri, Y.; O’Donohue, M.J. Biocatalytic conversion of wheat bran hydrolysate using an immobilized GH43 β-xylosidase. Bioresour. Technol. 2009, 100, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.; Gertler, A.; Hofmann, T. Aspergillus oryzae acid proteinase. Purification and properties, and formation of 7π-chymotrypsin. Biochem. J. 1975, 147, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Berger, B.; de Raadt, A.; Griengl, H.; Hayden, W.; Hechtberger, P.; Klempier, N.; Faber, K. Useful hydrolytic enzymes: Proteases, lipases and nitrilases. Pure Appl. Chem. 1992, 64, 1085–1088. [Google Scholar] [CrossRef]
- Theron, L.W.; Divol, B. Microbial aspartic proteases: Current and potential applications in industry. Appl. Microbiol. Biotechnol. 2014, 98, 8853–8868. [Google Scholar] [CrossRef] [PubMed]
- Pinelo, M.; Zeuner, B.; Meyer, A.S. Juice clarification by protease and pectinase treatments indicates new roles of pectin and protein in cherry juice turbidity. Food Bioprod. Process. 2010, 88, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Jeewanthi, R.K.C.; Lee, N.-K.; Lee, S.-K.; Yoon, Y.C.; Paik, H.-D. Physicochemical characterization of hydrolysates of whey protein concentrates for their use in nutritional beverages. Food Sci. Biotechnol. 2015, 24, 1335–1340. [Google Scholar]
- Waters, E.J.; Pellerin, P.; Brillouet, J.M. A wine arabinogalactanprotein that reduces heat-induced wine protein haze. Biosci. Biotechnol. Biochem. 1994, 58, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Peng, Z.; Pocock, K.F.; Williams, P.J. Proteins in white wine, I: Procyanidin occurrence in soluble proteins and insoluble protein hazes and its relationship to protein instability. Aust. J. Grape Wine Res. 1995, 1, 86–93. [Google Scholar] [CrossRef]
- Bell, S.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. In Advances in Wine Science; Blair, R., Francis, M., Pretorius, I., Eds.; The Australian Wine Research Institute: Adelaide, Australia, 2005; pp. 45–91. [Google Scholar]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Nelson, N. A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar]
- Somogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23. [Google Scholar]
- Collmer, A.; Ried, J.L.; Mount, M.S. Assay methods for pectic enzymes. Methods Enzymol. 1988, 161, 329–335. [Google Scholar]
- Guérin, L.; Sutter, D.-H.; Demois, A.; Chereau, M.; Trandafir, G. Determination of activity profiles of the main commercial enzyme preparations used in winemaking. Am. J. Enol. Vitic. 2009, 60, 322–331. [Google Scholar]
- Vico, I.; Jurick, W.M., II; Camp, M.J.; Januisiewicz, W.J.; Conway, W.S. Temperature suppresses decay on apple fruit by affecting Penicillium solitatum conidial germination, Mycelial growth, and Polygalacturonase activity. Plant Pathol. J. 2010, 9, 144–148. [Google Scholar]
- Mohsen, S.M.; Bazaraa, W.A.; Doukani, K. Purification and characterization of Aspergillus niger U-86 Polygalacturonase and its use in clarification of pomegranate and grape juices. In Proceedings of the 4th Conference on Recent Technologies in Agriculture, Cairo, Giza, Egypt, 3–5 November 2009; pp. 805–817. [Google Scholar]
- Anuradha, K.; Naga Padma, P.; Venkateshwar, S.; Reddy, G. Mango juice clarification with Polygalacturonase produced by Aspergillus awamori MTCC 9166-Optimization of conditions. Int. Food Res. J. 2016, 23, 147–151. [Google Scholar]
- Wang, S.; Lian, Z.; Wang, L.; Yang, X.; Liu, Y. Preliminary investigations on a Polygalacturonase from Aspergillus fumigatus in Chinese Púer tea fermentation. Biores. Bioprocess 2015, 2, 33–45. [Google Scholar] [CrossRef]
- Kant, S.; Vohra, A.; Gupta, R. Purification and physicochemical properties of Polygalacturonase from Aspergillus niger MTCC 3323. Protein Expr. Purif. 2013, 87, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Temple, D.; Ough, C.S. Inhibition of catalase in wines. Am. J. Enol. Vitic. 1975, 26, 92–96. [Google Scholar]
- Merín, M.G.; Martín, M.C.; Rantsiou, K.; Cocolin, L.; de Ambrosini, V.I.M. Characterization of pectinase activity for enology from yeasts occurring in Argentine Bonarda grape. Braz. J. Microbiol. 2015, 46, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.N.; Ramanan, R.N.; Mohamad, R.; Ariff, A.B. Improved mannan-degrading enzymes’ production by Aspergillus niger through medium optimization. New Biotechnol. 2011, 28, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, W.H.; Rosea, S.H.; Trollope, K.; Gorgens, J.F. Fungal-mannanases: Mannan hydrolysis, heterologous production and biotechnological applications. Process Biochem. 2010, 45, 1203–1213. [Google Scholar] [CrossRef]
- Pham, T.A.; Berrin, J.-G.; Record, E.; To, K.A.; Sigoillot, J.-C. Hydrolysis of softwood by Aspergillus mannanase: Role of a carbohydrate-binding module. J. Biotechnol. 2010, 148, 163–170. [Google Scholar] [CrossRef] [PubMed]
- De Vries, R.P.; van den Broeck, H.C.; Dekkers, E.; Manzanares, P.; de Graaff, L.H.; Visser, J. Differential expression of three α-galactosidase genes and a single β-galactosidase gene from Aspergillus niger. Appl. Environ. Microbiol. 1999, 65, 2453–2460. [Google Scholar] [PubMed]
- Courtois, J.E.; Petek, F. α-Galactosidase from coffee beans. Methods Enzymol. 1966, 8, 565–571. [Google Scholar]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, A.G.; Knox, A.; Di Profio, F. Evaluation of Macerating Pectinase Enzyme Activity under Various Temperature, pH and Ethanol Regimes. Beverages 2018, 4, 10. https://doi.org/10.3390/beverages4010010
Reynolds AG, Knox A, Di Profio F. Evaluation of Macerating Pectinase Enzyme Activity under Various Temperature, pH and Ethanol Regimes. Beverages. 2018; 4(1):10. https://doi.org/10.3390/beverages4010010
Chicago/Turabian StyleReynolds, Andrew G., Anthony Knox, and Frederick Di Profio. 2018. "Evaluation of Macerating Pectinase Enzyme Activity under Various Temperature, pH and Ethanol Regimes" Beverages 4, no. 1: 10. https://doi.org/10.3390/beverages4010010
APA StyleReynolds, A. G., Knox, A., & Di Profio, F. (2018). Evaluation of Macerating Pectinase Enzyme Activity under Various Temperature, pH and Ethanol Regimes. Beverages, 4(1), 10. https://doi.org/10.3390/beverages4010010