Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes
Abstract
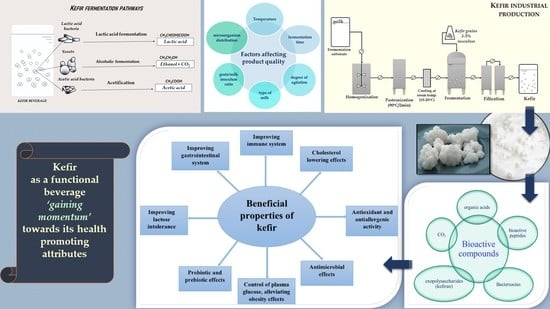
:1. Historical Pathway of Fermented Milk in Human Nutrition
2. Health-Promoting Effects of Kefir
2.1. Nutritional Characteristics of Kefir
2.2. Kefir Microbial Diversity and Beneficial Value
2.3. Kefir Beverages as Protective Dietary Supplements against Viral Infections
3. Commercial Kefir Production
Quality Production Management
4. Modern Biotechnological Processes and Applications
5. Future Prospects towards Health-Promoting Effects
Funding
Conflicts of Interest
References
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bats, A. The production of bread in conical moulds at the beginning of the Egyptian Middle Kingdom: The contribution of experimental archaeology. J. Archaeol. Sci. Rep. 2020, 34, 102631. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Rosenberg, D.; Zhao, H.; Lengyel, G.; Nadel, D. Response to comments on archaeological reconstruction of 13,000-y old Natufian beer making at Raqefet Cave, Israel. J. Archaeol. Sci. Rep. 2019, 28, 101914. [Google Scholar] [CrossRef]
- Gest, H. The discovery of microorganisms by Robert Hooke and Antoni van Leeuwenhoek, fellows of the Royal Society. Notes Rec. R. Soc. 2004, 58, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Turkmen, N. Chapter 29—Kefir as a Functional Dairy Product. In Dairy in Human Health and Disease Across the Lifespan; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 373–383. [Google Scholar]
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Abd El-Salam, M.H. Fermented Milks—Middle Eastern Fermented Milks. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 503–506. [Google Scholar]
- Terpou, A. Ethnic Selected Fermented Foods of Greece. In Fermented Food Products, 1st ed.; Sankaranarayanan, N.A., Dhanasekaran, D., Eds.; CRC Press: London, UK; Taylor & Francis Group: New York, NY, USA, 2020; p. 10. [Google Scholar]
- Ouwehand, A.C. Probiotics: Time to move beyond Metchnikoff? Drug Discov. Today 2003, 8, 1063. [Google Scholar] [CrossRef]
- Baud, D.; Dimopoulou Agri, V.; Gibson, G.R.; Reid, G.; Giannoni, E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 2020, 8, 186. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Bezirtzoglou, E. Probiotics as a Weapon in the Fight Against COVID-19. Front. Nutr. 2020, 7. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Gupta, A.M.; Mitra, S.; Mandal, S.; Biswas, S.R. A natural food preservative peptide nisin can interact with the SARS-CoV-2 spike protein receptor human ACE2. Virology 2021, 552, 107–111. [Google Scholar] [CrossRef]
- Chourasia, R.; Padhi, S.; Chiring Phukon, L.; Abedin, M.M.; Singh, S.P.; Rai, A.K. A Potential Peptide from Soy Cheese Produced Using Lactobacillus delbrueckii WS4 for Effective Inhibition of SARS-CoV-2 Main Protease and S1 Glycoprotein. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Gialleli, A.-I.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Kanellaki, M. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod. Process. 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Terpou, A.; Bosnea, L.; Kanellaki, M.; Plessas, S.; Bekatorou, A.; Bezirtzoglou, E.; Koutinas, A.A. Growth Capacity of a Novel Potential Probiotic Lactobacillus paracasei K5 Strain Incorporated in Industrial White Brined Cheese as an Adjunct Culture. J. Food Sci. 2018, 83, 723–731. [Google Scholar] [CrossRef] [PubMed]
- de Simone, C. The Unregulated Probiotic Market. Clin. Gastroenterol. Hepatol. 2019, 17, 809–817. [Google Scholar] [CrossRef] [Green Version]
- Terpou, A.; Mantzourani, I. Vinegars Made with Kefir. In Advances in Vinegar Production, 1st ed.; Bekatorou, A., Ed.; CRC Press: London, UK; Taylor & Francis Group: New York, NY, USA, 2019; p. 16. [Google Scholar]
- Guzel-Seydim, Z.B.; Gokirmaklı, C.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Jomaa, S.A.; Abd El-Wahed, A.; El-Seedi, H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef] [Green Version]
- Hong, W.S.; Chen, Y.P.; Chen, M.J. The antiallergic effect of kefir lactobacilli. J. Food Sci. 2010, 75, H244–H253. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, R.S.; Lopes, G.A.C.; Ferreira, N.S.; Pinto, E.P.; Carvalho, J.C.T.; Figueiredo, S.S.; Oliveira, A.F.; Zamora, R.R.M. Superficial Characterization of Kefir Biofilms Associated with Açaí and Cupuaçu Extracts. Arab. J. Sci. Eng. 2018, 43, 3371–3379. [Google Scholar] [CrossRef]
- Hikmetoglu, M.; Sogut, E.; Sogut, O.; Gokirmakli, C.; Guzel-Seydim, Z.B. Changes in carbohydrate profile in kefir fermentation. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100220. [Google Scholar] [CrossRef]
- de Oliveira Leite, A.M.; Miguel, M.A.L.; Peixoto, R.S.; Rosado, A.S.; Silva, J.T.; Paschoalin, V.M.F. Microbiological, technological and therapeutic properties of kefir: A natural probiotic beverage. Braz. J. Microbiol. 2013, 44, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.P.D.; Santos, G.A.D.; Nomura, C.S.; Naozuka, J. Elemental chemical composition of products derived from kefir fermented milk. J. Food Compos. Anal. 2019, 78, 86–90. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms 2021, 9, 480. [Google Scholar] [CrossRef]
- Garofalo, C.; Osimani, A.; Milanovic, V.; Aquilanti, L.; de Filippis, F.; Stellato, G.; di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef]
- Dertli, E.; Con, A.H. Microbial diversity of traditional kefir grains and their role on kefir aroma. LWT Food Sci. Technol. 2017, 85, 151–157. [Google Scholar] [CrossRef]
- Rodrigues, K.L.; Caputo, L.R.G.; Carvalho, J.C.T.; Evangelista, J.; Schneedorf, J.M. Antimicrobial and healing activity of kefir and kefiran extract. Int. J. Antimicrob. Agents 2005, 25, 404–408. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Leite, D.C.A.; Del Aguila, E.M.; Alvares, T.S.; Peixoto, R.S.; Miguel, M.A.L.; Silva, J.T.; Paschoalin, V.M.F. Microbiological and chemical characteristics of Brazilian kefir during fermentation and storage processes. J. Dairy Sci. 2013, 96, 4149–4159. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Athanasiadis, I.; Bekatorou, A.; Psarianos, C.; Kanellaki, M.; Agouridis, N.; Blekas, G. Kefir-yeast technology: Industrial scale-up of alcoholic fermentation of whey, promoted by raisin extracts, using kefir-yeast granular biomass. Enzym. Microb. Technol. 2007, 41, 576–582. [Google Scholar] [CrossRef]
- Miguel, M.G.d.C.P.; Cardoso, P.G.; Lago, L.d.A.; Schwan, R.F. Diversity of bacteria present in milk kefir grains using culture-dependent and culture-independent methods. Food Res. Int. 2010, 43, 1523–1528. [Google Scholar] [CrossRef]
- Nalbantoglu, U.; Cakar, A.; Dogan, H.; Abaci, N.; Ustek, D.; Sayood, K.; Can, H. Metagenomic analysis of the microbial community in kefir grains. Food Microbiol. 2014, 41, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bourrie, B.C.T.; Cotter, P.D.; Willing, B.P. Traditional kefir reduces weight gain and improves plasma and liver lipid profiles more successfully than a commercial equivalent in a mouse model of obesity. J. Funct. Foods 2018, 46, 29–37. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Yüksekdağ, Z.N.; Beyatli, Y.; Aslim, B. Determination of some characteristics coccoid forms of lactic acid bacteria isolated from Turkish kefirs with natural probiotic. LWT Food Sci. Technol. 2004, 37, 663–667. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, D.-H.; Kang, I.-B.; Kim, H.; Song, K.-Y.; Kim, H.-S.; Seo, K.-H. Characterization and antibacterial activity of a novel exopolysaccharide produced by Lactobacillus kefiranofaciens DN1 isolated from kefir. Food Control. 2017, 78, 436–442. [Google Scholar] [CrossRef]
- Londero, A.; Hamet, M.F.; De Antoni, G.L.; Garrote, G.L.; Abraham, A.G. Kefir grains as a starter for whey fermentation at different temperatures: Chemical and microbiological characterisation. J. Dairy Res. 2012, 79, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Korsak, N.; Taminiau, B.; Leclercq, M.; Nezer, C.; Crevecoeur, S.; Ferauche, C.; Detry, E.; Delcenserie, V.; Daube, G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015, 98, 3684–3689. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, C.; Ferrocino, I.; Reale, A.; Sabbatini, R.; Milanović, V.; Alkić-Subašić, M.; Boscaino, F.; Aquilanti, L.; Pasquini, M.; Trombetta, M.F.; et al. Study of kefir drinks produced by backslopping method using kefir grains from Bosnia and Herzegovina: Microbial dynamics and volatilome profile. Food Res. Int. 2020, 137, 109369. [Google Scholar] [CrossRef]
- Sindi, A.; Badsha, M.B.; Unlu, G. Bacterial Populations in International Artisanal Kefirs. Microorganisms 2020, 8, 1318. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Jiang, H.; Dong, M. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Rakshit, S.K.; Pagnoncelli, M.G.B.; de Souza Vandenberghe, L.P.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.O.; Magalhães-Guedes, K.T.; Braga, R.A.; Dias, D.R.; Schwan, R.F. Fermentation process for production of apple-based kefir vinegar: Microbiological, chemical and sensory analysis. Braz. J. Microbiol. 2017, 48, 592–601. [Google Scholar] [CrossRef]
- Chen, M.-J.; Tang, H.-Y.; Chiang, M.-L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ahmed, Z.; Feng, W.; Li, C.; Song, S. Physicochemical properties of exopolysaccharide produced by Lactobacillus kefiranofaciens ZW3 isolated from Tibet kefir. Int. J. Biol. Macromol. 2008, 43, 283–288. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Mayo, B.; Rachid, C.T.C.C.; Peixoto, R.S.; Silva, J.T.; Paschoalin, V.M.F.; Delgado, S. Assessment of the microbial diversity of Brazilian kefir grains by PCR-DGGE and pyrosequencing analysis. Food Microbiol. 2012, 31, 215–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Fang, H.; Padhiar, A.A.; Zeng, X.; Khurshid, M.; He, Z.; Zhao, L. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J. Funct. Foods 2019, 63, 103588. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Medeiros, A.P.; Rakshit, S.K.; Soccol, C.R. Development of kefir-based probiotic beverages with DNA protection and antioxidant activities using soybean hydrolyzed extract, colostrum and honey. LWT Food Sci. Technol. 2016, 68, 690–697. [Google Scholar] [CrossRef]
- Bengoa, A.A.; Iraporda, C.; Acurcio, L.B.; de Cicco Sandes, S.H.; Costa, K.; Moreira Guimarãaes, G.; Esteves Arantes, R.M.; Neumann, E.; Cantini Nunes, A.; Nicoli, J.R.; et al. Physicochemical, immunomodulatory and safety aspects of milks fermented with Lactobacillus paracasei isolated from kefir. Food Res. Int. 2019, 123, 48–55. [Google Scholar] [CrossRef]
- Gangoiti, M.V.; Puertas, A.I.; Hamet, M.F.; Peruzzo, P.J.; Llamas, M.G.; Medrano, M.; Prieto, A.; Duenas, M.T.; Abraham, A.G. Lactobacillus plantarum CIDCA 8327: An α-glucan producing-strain isolated from kefir grains. Carbohydr. Polym. 2017, 170, 52–59. [Google Scholar] [CrossRef]
- Kakisu, E.; Bolla, P.; Abraham, A.G.; de Urraza, P.; De Antoni, G.L. Lactobacillus plantarum isolated from kefir: Protection of cultured Hep-2 cells against Shigella invasion. Int. Dairy J. 2013, 33, 22–26. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Kim, D.-H.; Jeong, D.; Seo, K.-H.; Jeong, H.S.; Lee, H.G.; Kim, H. Characterization of yeasts isolated from kefir as a probiotic and its synergic interaction with the wine byproduct grape seed flour/extract. LWT 2018, 90, 535–539. [Google Scholar] [CrossRef]
- de Paiva, I.M.; da Silva Steinberg, R.; Lula, I.S.; de Souza-Fagundes, E.M.; de Oliveira Mendes, T.; Bell, M.J.V.; Nicoli, J.R.; Nunes, A.C.; Neumann, E. Lactobacillus kefiranofaciens and Lactobacillus satsumensis isolated from Brazilian kefir grains produce alpha-glucans that are potentially suitable for food applications. LWT Food Sci. Technol. 2016, 72, 390–398. [Google Scholar] [CrossRef]
- Deng, Y.; Man, C.; Fan, Y.; Wang, Z.; Li, L.; Ren, H.; Cheng, W.; Jiang, Y. Preparation of elemental selenium-enriched fermented milk by newly isolated Lactobacillus brevis from kefir grains. Int. Dairy J. 2015, 44, 31–36. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grzeskowiak, L.M.; Reis, S.A.; Conceiçao, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.; Ryan, A.; Hudson, S.P. Pre-Formulation and Delivery Strategies for the Development of Bacteriocins as Next Generation Antibiotics. Eur. J. Pharm. Biopharm. 2021. [Google Scholar] [CrossRef]
- Verma, J.; Subbarao, N. A comparative study of human betacoronavirus spike proteins: Structure, function and therapeutics. Arch. Virol. 2021, 166, 697–714. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential beneficial role of probiotics on the outcome of COVID-19 patients: An evolving perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Daba, G.M.; Elkhateeb, W.A. Bacteriocins of lactic acid bacteria as biotechnological tools in food and pharmaceuticals: Current applications and future prospects. Biocatal. Agric. Biotechnol. 2020, 28, 101750. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Zawawi, N.; Abdull Razis, A.F.; Bakar, J.; Zarei, M. Antiviral activity of fermented foods and their probiotics bacteria towards respiratory and alimentary tracts viruses. Food Control. 2021, 127, 108140. [Google Scholar] [CrossRef]
- Balmeh, N.; Mahmoudi, S.; Fard, N.A. Manipulated bio antimicrobial peptides from probiotic bacteria as proposed drugs for COVID-19 disease. Inform. Med. Unlocked 2021, 23, 100515. [Google Scholar] [CrossRef]
- Małaczewska, J.; Kaczorek-Łukowska, E. Nisin—A lantibiotic with immunomodulatory properties: A review. Peptides 2021, 137, 170479. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hilphy, A.R.; Abdulstar, A.R.; Gavahian, M. Moderate electric field pasteurization of milk in a continuous flow unit: Effects of process parameters, energy consumption, and shelf-life determination. Innov. Food Sci. Emerg. Technol. 2021, 67, 102568. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Guimarães, J.T.; Cruz, A.G.; Sant’Ana, A.S. Hazards of a ‘healthy’ trend? An appraisal of the risks of raw milk consumption and the potential of novel treatment technologies to serve as alternatives to pasteurization. Trends Food Sci. Technol. 2018, 82, 148–166. [Google Scholar] [CrossRef]
- O’Brien, K.V.; Aryana, K.J.; Prinyawiwatkul, W.; Ordonez, K.M.C.; Boeneke, C.A. Short communication: The effects of frozen storage on the survival of probiotic microorganisms found in traditionally and commercially manufactured kefir. J. Dairy Sci. 2016, 99, 7043–7048. [Google Scholar] [CrossRef] [Green Version]
- Savastano, M.L.; Pati, S.; Bevilacqua, A.; Corbo, M.R.; Rizzuti, A.; Pischetsrieder, M.; Losito, I. Influence of the production technology on kefir characteristics: Evaluation of microbiological aspects and profiling of phosphopeptides by LC-ESI-QTOF-MS/MS. Food Res. Int. 2020, 129, 108853. [Google Scholar] [CrossRef]
- Moonga, H.B.; Schoustra, S.E.; Linnemann, A.R.; van den Heuvel, J.; Shindano, J.; Smid, E.J. Influence of fermentation temperature on microbial community composition and physicochemical properties of mabisi, a traditionally fermented milk. LWT 2021, 136, 110350. [Google Scholar] [CrossRef]
- Noğay, N.H. 8—Kefir Beverage and Its Effects on Health. In Milk-Based Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 273–296. [Google Scholar]
- Walsh, A.M.; Crispie, F.; Kilcawley, K.; O’Sullivan, O.; O’Sullivan, M.G.; Claesson, M.J.; Cotter, P.D. Microbial Succession and Flavor Production in the Fermented Dairy Beverage Kefir. mSystems 2016, 1. [Google Scholar] [CrossRef] [Green Version]
- Exarhopoulos, S.; Raphaelides, S.N.; Kontominas, M.G. Flow behavior studies of kefiran systems. Food Hydrocoll. 2018, 79, 282–290. [Google Scholar] [CrossRef]
- Kesenkas, H.; Gursoy, O.; Ozbaş, H. Chapter 14—Kefir A2—Frias, Juana. In Fermented Foods in Health and Disease Prevention; Martinez-Villaluenga, C., Penas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 339–361. [Google Scholar]
- Laureys, D.; Aerts, M.; Vandamme, P.; De Vuyst, L. Oxygen and diverse nutrients influence the water kefir fermentation process. Food Microbiol. 2018, 73, 351–361. [Google Scholar] [CrossRef]
- de la Fuente-Salcido, N.M.; Castañeda-Ramírez, J.C.; García-Almendárez, B.E.; Bideshi, D.K.; Salcedo-Hernández, R.; Barboza-Corona, J.E. Isolation and characterization of bacteriocinogenic lactic bacteria from M-Tuba and Tepache, two traditional fermented beverages in México. Food Sci. Nutr. 2015, 3, 434–442. [Google Scholar] [CrossRef]
- Gaware, V.; Kotade, K.; Dolas, R.; Dhamak, K. The magic of kefir: A review. Pharmacology 2011, 1, 376–386. [Google Scholar]
- Yépez, A.; Russo, P.; Spano, G.; Khomenko, I.; Biasioli, F.; Capozzi, V.; Aznar, R. In situ riboflavin fortification of different kefir-like cereal-based beverages using selected Andean LAB strains. Food Microbiol. 2019, 77, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Koutinas, A.A.; Papapostolou, H.; Dimitrellou, D.; Kopsahelis, N.; Katechaki, E.; Bekatorou, A.; Bosnea, L.A. Whey valorisation: A complete and novel technology development for dairy industry starter culture production. Bioresour. Technol. 2009, 100, 3734–3739. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Corona, O.; Randazzo, W.; Miceli, A.; Guarcello, R.; Francesca, N.; Erten, H.; Moschetti, G.; Settanni, L. Characterization of kefir-like beverages produced from vegetable juices. LWT Food Sci. Technol. 2016, 66, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Kourkoutas, Y.; Kandylis, P.; Panas, P.; Dooley, J.S.G.; Nigam, P.; Koutinas, A.A. Evaluation of freeze-dried kefir coculture as starter in feta-type cheese production. Appl. Environ. Microbiol. 2006, 72, 6124–6135. [Google Scholar] [CrossRef] [Green Version]
- Altuntas, S.; Hapoglu, H. 7—Kefir-Type Drinks from Whey. In Non-Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 185–226. [Google Scholar]
- Hatmal, M.m.M.; Nuirat, A.; Zihlif, M.A.; Taha, M.O. Exploring the influence of culture conditions on kefir’s anticancer properties. J. Dairy Sci. 2018, 101, 3771–3777. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Jeong, D.; Song, K.-Y.; Seo, K.-H. Comparison of traditional and backslopping methods for kefir fermentation based on physicochemical and microbiological characteristics. LWT 2018, 97, 503–507. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of antioxidant capacity of cow and ewe milk kefirs. J. Dairy Sci. 2018, 101, 3788–3798. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.K.; Park, E.J.; Ko, S.Y.; Choi, E.W.; Kim, S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2,4,6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. J. Dairy Sci. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Chen, H.-L.; Fan, H.-C.; Tung, Y.-T.; Kuo, C.-W.; Tu, M.-Y.; Chen, C.-M. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats. Antioxidants 2020, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Gamba, R.R.; Caro, C.A.; Martínez, O.L.; Moretti, A.F.; Giannuzzi, L.; De Antoni, G.L.; León Peláez, A. Antifungal effect of kefir fermented milk and shelf life improvement of corn arepas. Int. J. Food Microbiol. 2016, 235, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. Anti-diabetes functionality of Kefir culture-Mediated fermented soymilk supplemented with Rhodiola extracts. Food Biotechnol. 2006, 20, 13–29. [Google Scholar] [CrossRef]
- Barboza, K.R.M.; Coco, L.Z.; Alves, G.M.; Peters, B.; Vasquez, E.C.; Pereira, T.M.C.; Meyrelles, S.S.; Campagnaro, B.P. Gastroprotective effect of oral kefir on indomethacin-induced acute gastric lesions in mice: Impact on oxidative stress. Life Sci. 2018, 209, 370–376. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Clancy, S.M. Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. J. Am. Diet. Assoc. 2003, 103, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Shami, A.; Ali, M.A.; Almohawes, Z.N.; Mohammed, A.E.; Bin-Meferij, M.M. Kefir: A protective dietary supplementation against viral infection. Biomed. Pharmacother. 2021, 133, 110974. [Google Scholar] [CrossRef]
- Vinderola, G.; Perdigon, G.; Duarte, J.; Thangavel, D.; Farnworth, E.; Matar, C. Effects of kefir fractions on innate immunity. Immunobiology 2006, 211, 149–156. [Google Scholar] [CrossRef]



| Microbial Species | Reference | Microbial Species | Reference |
|---|---|---|---|
| Lactobacilli | Lactococci | ||
| Furfurilactobacillus rossiae | [36,38] | Lactococcus cremoris | [39] |
| Lactobacillus acidophilus | [36,38,40,41] | Lactococcus garvieae | [36] |
| Lactobacillus amylovorus | [36,38,40] | Lactococcus lactis subsp. lactis | [30,33,41,42,43] |
| Lactobacillus apis | [31,38,44] | Lactococcus lactis subsp. cremoris | [33,41,45] |
| Lactobacillus bulgaricus | [38,40] | Streptococci | |
| Lacticaseibacillus casei | [36,38,40] | Streptococcus durans | [39] |
| Lactobacillus crispatus | [36,38,40,41] | Streptococcus faecalis | [39] |
| Lactobacillus delbrueckii subsp. | [36,38] | Streptococcus thermophilus | [30,39] |
| Lactobacillus fomicalis | [38,40] | Acetic acid bacteria | |
| Lactobacillus gallinarum | [36,38,40] | Acetobacter aceti | [46] |
| Lactobacillus gasseri | [38,40] | Acetobacter fabarum | [30] |
| Lactobacillus gigeriorum | [44] | Acetobacter genera | [30] |
| Lactobacillus helveticus | [35,38,40,45] | Acetobacter lovaniensis | [30,42] |
| Lactobacillus intestinalis | [38,40] | Acetobacter orientalis | [30,42] |
| Lactobacillus jensenii | [38,41] | Acetobacter orleanensis | [44] |
| Lactobacillus kalixensis | [38,40] | Acetobacter pasteurianus | [44,47] |
| Lactobacillus kefiranofaciens subsp. kefiranofaciens | [30,31,33,38,40,41,43,44,48,49] | Acetobacter syzygii | [35,43,47] |
| Lactobacillus kefiranofaciens subsp. kefirgranum | [38,50] | Gluconobacter japonicus | [35] |
| Lentilactobacillus kefiri | [30,31,38,41,44,50,51] | Gluconobacter morbifer | [44] |
| Lactobacillus kitasatonis | [38,40] | Yeast | |
| Lactobacillus mesenteroides | [38,45] | Kazachstania aquatica | [41] |
| Lentilactobacillus otakiensis | [30,38] | Dekkera anomala | [30] |
| Lentilactobacillus parabuchneri | [38,50] | Hanseniaspora uvarum | [52] |
| Lactobacillus paracasei subsp. paracasei | [35,38,47,53] | Issatchenkia orientalis | [52] |
| Lentilactobacillus parakefiri | [35,38] | Kazachstania aerobia | [30] |
| Lactiplantibacillus pentosus | [36,38] | Kazachstania exigua | [42] |
| Lactiplantibacillus plantarum subsp. plantarum | [35,38,47,54,55] | Kazachstania servazzii | [30] |
| Limosilactobacillus reuteri | [36,38] | Kazachstania solicola | [30] |
| Lacticaseibacillus rhamnosus | [36,38] | Kazachstania turicensis | [30,41] |
| Lactobacillus rodentium | [38,40] | Kazachstania unispora | [30,41,43,45] |
| Latilactobacillus sakei subsp. sakei | [36,38] | Kluyveromyces lactis | [45,56] |
| Ligilactobacillus salivarius | [36,38] | Kluyveromyces marxianus | [41,42,43,56] |
| Liquorilactobacillus satsumensis | [38,57] | Pichia fermentans | [43] |
| Lentilactobacillus sunkii | [30,38] | Pichia membranifaciens | [52] |
| Lactobacillus ultunensis | [38,40,44] | Pichia kudriavzevii | [52] |
| Levilactobacillus brevis | [36,38,58] | Saccharomyces cariocanus | [30] |
| Liquorilactobacillus uvarum | [35,38] | Saccharomyces cerevisiae | [30,43,47,52,56] |
| Lentilactobacillus buchneri subsp. | [30,38] | Saccharomyces servazzii | [41] |
| Other bacteria | Zygosaccharomyces fermentati | [52] | |
| Pediococcus halophilus | [36] | Other bacteria | |
| Pediococcus lolii | [36] | Enterococcus sp. | [30] |
| Pediococcus pentosaceus | [36] | Leuconostoc mesenteroides | [33,52] |
| Lysinibacillus sphaericus | [52] | Leuconostoc pseudomesenteroides | [43] |
| Microbial Groups | Effect on Kefir Beverage |
|---|---|
| Mesophilous lactic acid Streptococci(Streptococcus lactis, Streptococcus cremoris) | Development of acidification and coagulate formation. |
| Lactic acid bacteria (Lactobacillus kefiranofaciens subsp. kefiranofaciens, Lentilactobacillus kefiri, Lactiplantibacillus plantarum subsp. plantarum, Lactobacillus helveticus ) Lactococci (Lactococcus lactis, Streptococcus diacetylactis, Leuconostoc dextranicum) | Development of sour taste and lower pH. Stabilization of kefiran structure in kefir grains. Affects the rheological characteristics of the final product. Production of volatile and other compounds with positive effects on texture and flavor. |
| Yeasts (Saccharomyces cerevisiae, Saccharomyces unisporus, Kluyveromyces marxianus) | Development of a slight alcoholic flavor to the final product. Provide a mildly carbonated effect due to CO2 formation. |
| Acetic acid bacteria | No significant effect. Excessive growth can cause viscous consistency. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganatsios, V.; Nigam, P.; Plessas, S.; Terpou, A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages 2021, 7, 48. https://doi.org/10.3390/beverages7030048
Ganatsios V, Nigam P, Plessas S, Terpou A. Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages. 2021; 7(3):48. https://doi.org/10.3390/beverages7030048
Chicago/Turabian StyleGanatsios, Vassilios, Poonam Nigam, Stavros Plessas, and Antonia Terpou. 2021. "Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes" Beverages 7, no. 3: 48. https://doi.org/10.3390/beverages7030048
APA StyleGanatsios, V., Nigam, P., Plessas, S., & Terpou, A. (2021). Kefir as a Functional Beverage Gaining Momentum towards Its Health Promoting Attributes. Beverages, 7(3), 48. https://doi.org/10.3390/beverages7030048