Natural Intervarietal Hybrids of Coffea canephora Have a High Content of Diterpenes
Abstract
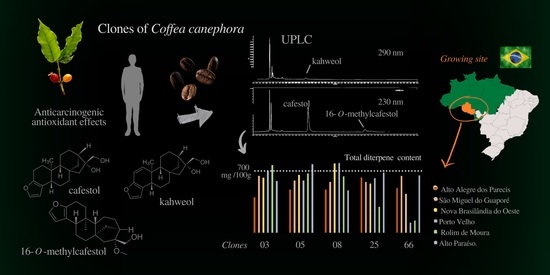
:1. Introduction
2. Materials and Methods
2.1. Reagents, Standards, and Equipment
2.2. Material
2.3. Diterpene Analysis
2.4. Data Analysis
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ICO International Coffee Organization. Trade Statistics Tables. Available online: https://www.ico.org/trade_statistics.asp?section=Statistics (accessed on 21 October 2021).
- MAPA Ministério da Agricultura, Pecuária e Abastecimento/Secretaria de Política Agrícola. Sumário Executivo Café Abril–2021. Available online: https://www.gov.br/agricultura/pt–br/assuntos/politica–agricola/todas–publicacoes–de–politica–agricola/sumarios–executivos–de–produtos–agricolas/cafe–pdf/view (accessed on 21 October 2021).
- Fiorott, A.S.; Sturm, G.M. Café Canéfora: Em busca de qualidade e reconhecimento. In Café na Amazônia, 1st ed.; Marcolan, A.L., Espindula, M.C., Eds.; Embrapa: Brasiliaa, Brazil, 2015; Volume 1, pp. 425–431. [Google Scholar]
- Teixeira, A.L.; Rocha, R.B.; Espindula, M.C.; Ramalho, A.R.; Vieira Júnior, J.R.; Alves, E.A.; Lunz, A.M.P.; Souza, F.F.; Costa, J.N.M.; Fernandes, C.F. Amazonian Robustas—New Coffea canephora coffee cultivars for the Western Brazilian Amazon. Crop. Breed. Appl. Biotechnol. 2020, 20, 1–5. [Google Scholar] [CrossRef]
- Espindula, M.C.; Dias, J.R.M.; Rocha, R.B.; Dalazen, J.R.; De Araújo, L.V. Café em Rondônia. In Café Conilon: Gestão e Manejo Com Sustentabilidade, 1st ed.; Partelli, F.L., Gontijo, I., Eds.; Caufes: Alegre, Brazil, 2017; Volume 1, pp. 69–88. [Google Scholar]
- Dalazen, J.R.; Rocha, R.B.; Espindula, M.C.; Dias, J.R.M.; Dalazen, J.R. Base genética da cafeicultura e caracterização dos principais clones cultivados no estado de Rondônia. In Café Conilon: Conhecimento Para Superar Desafios, 1st ed.; Partelli, F.L., Espindula, M.C., Eds.; Caufes: Alegre, Brazil, 2019; Volume 1, pp. 165–177. [Google Scholar]
- Ferrão, L.F.V.; Caixeta, E.T.; Souza, F.F.; Zambolim, E.M.; Cruz, C.D.; Zambolim, L.; Sakiyama, N.S. Comparative study of different molecular markers for classifying and establishing genetic relationships in Coffea canephora. Plant Syst. Evol. 2013, 299, 225–238. [Google Scholar] [CrossRef]
- Montagnon, C.; Cubry, P.; Leroy, T. Amélioration génétique du caféier Coffea canephora Pierre: Connaissances acquises, stratégies et perspectives. Cah. Agric. 2012, 21, 143–153. [Google Scholar]
- Souza, F.F.; Ferrão, L.F.V.; Caixeta, E.T.; Sakiyama, N.S.; Pereira, A.A.; De Oliveira, A.C.B. Aspectos gerais da biologia e da diversidade genética de Coffea canéfora. In Café na Amazônia, 1st ed.; Marcolan, A.L., Espindula, M.C., Eds.; Embrapa: Brasilia, Brazil, 2015; Volume 1, pp. 83–98. [Google Scholar]
- Dalazen, J.R.; Rocha, R.B.; Pereira, L.L.; Alves, E.A.; Espindula, M.C.; Souza, C.A. Beverage quality of most cultivated Coffea canephora clones in the Western Amazon. Coffee Sci. 2020, 15, e151711. [Google Scholar]
- Ramalho, A.R.; Rocha, R.B.; Souza, F.F.; Veneziano, W.; Teixeira, A.L. Progresso genético da produtividade de café beneficiado com a seleção de clones de cafeeiro ‘Conilon’. Rev. Ciênc. Agron. 2016, 47, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Dalcomo, J.M.; Vieira, H.D.; Ferreira, A.; Lima, W.L.; Ferrão, R.G.; Fonseca, A.F.A.; Ferrão, M.A.G.; Partelli, F.L. Evaluation of genetic divergence among clones of conilon coffee after scheduled cycle pruning. Genet. Mol. Res. 2015, 14, 15417–15426. [Google Scholar] [CrossRef] [PubMed]
- Rocha, R.B.; Teixeira, A.L.; Ramalho, A.R.; Souza, F.F. Melhoramento de Coffea canephora—Considerações e metodologias. In Café na Amazônia, 1st ed.; Marcolan, A.L., Espindula, M.C., Eds.; Embrapa: Brasilia, Brazil, 2015; Volume 1, pp. 99–126. [Google Scholar]
- Gӧkcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef] [Green Version]
- O'Keefe, J.H.; DiNicolantonio, J.J.; Lavie, C.J. Coffee for cardioprotection and longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butt, M.S.; Sultan, M.T. Coffee and its consumption: Benefits and risks. Crit. Rev. Food Sci. Nutr. 2018, 51, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Gaascht, F.; Dicato, M.; Diederich, M. Coffee provides a natural multitarget pharmacopeia against the hallmarks of cancer. Genes Nutr. 2015, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Alves, A. New Trends in coffee diterpenes research from technological to health aspects. Food Res. Int. 2020, 134, e109207. [Google Scholar] [CrossRef] [PubMed]
- Muriel, P.; Arauz, J. Coffee and liver diseases. Fitoterapia 2010, 81, 297–305. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, C.; Xu, J.; Wang, S. Cafestol and kahweol: A review on their bioactivities and pharmacological properties. Int. J. Mol. Sci. 2019, 20, 4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urgert, R.; Katan, M.B. The cholesterol–raising factor from coffee beans. J. R. Soc. Med. 1996, 89, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Benassi, M.T.; Dias, R.C.E. Assay of kahweol and cafestol in coffee. In Coffee in Health and Disease Prevention, 1st ed.; Preedy, V.R., Ed.; Elsevier: London, UK, 2015; Volume 1, pp. 993–1004. [Google Scholar]
- Campanha, F.G.; Dias, R.C.E.; Benassi, M.T. Discrimination of coffee species using kahweol and cafestol: Effects of roasting and of defects. Coffee Sci. 2010, 5, 87–96. [Google Scholar]
- Dias, R.C.E.; De Faria–Machado, A.F.; Mercadante, A.Z.; Bragagnolo, N.; Benassi, M.T. Roasting process affects the profile of diterpenes in coffee. Eur. Food Res. Technol. 2014, 239, 961–970. [Google Scholar] [CrossRef]
- De Souza, R.M.N.; Benassi, M.T. Discrimination of commercial roasted and ground coffees according to chemical composition. J. Braz. Chem. Soc. 2012, 23, 1347–1354. [Google Scholar] [CrossRef] [Green Version]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Mori, A.L.B.; Kalschne, D.L.; Ferrão, M.A.G.; Fonseca, A.F.A.; Ferrão, R.G.; Benassi, M.T. Diterpenes in Coffea canephora. J. Food Compos. Anal. 2016, 52, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Finotello, C.; Forzato, C.; Gasparini, A.; Mammi, S.; Navarini, L.; Schievano, E. NMR quantification of 16–O–methylcafestol and kahweol in Coffea canephora var. robusta beans from different geographical origins. Food Control. 2017, 75, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Gunning, Y.; Defernez, M.; Watson, A.D.; Beadman, N.; Colquhoun, I.J.; Gall, G.L.; Philo, M.; Garwood, H.; Williamson, D.; Davis, A.P.; et al. 16–O–methylcafestol is present in ground roast Arabica coffees: Implications for authenticity testing. Food Chem. 2018, 248, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Guercia, E.; Colomban, S.; Navarini, L. 16–O–Methylated diterpenes in green Coffea arabica: Ultra–high–performance liquid chromatography–tandem mass spectrometry method optimization and validation. J. Mass Spectrom. 2020, 55, e4636. [Google Scholar] [CrossRef] [PubMed]
- Portaluri, V.; Thomas, F.; Guyader, S.; Jamin, E.; Bertrand, B.; Remaud, G.S.; Schievano, E.; Mammi, S.; Guercia, E.; Navarini, L. Limited genotypic and geographic variability of 16–O–methylated diterpene content in Coffea arabica green beans. Food Chem. 2020, 329, 127129. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Scholz, M.B.S.; Pereira, L.F.P.; Vieira, L.G.E.; Sera, T.; Silva, J.B.G.D.; Benassi, M.T. Diterpenes in green and roasted coffee of Coffea arabica cultivars growing in the same edapho–climatic conditions. J. Food Compos. Anal. 2013, 30, 52–57. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Kitzberger, C.S.G.; Pagiatto, N.F.; Pereira, L.F.P.; Davrieux, F.; Pot, D.; Charmetant, P.; Thierry, L. Chemical composition in wild ethiopian Arabica coffee accessions. Euphytica 2016, 209, 429–438. [Google Scholar] [CrossRef]
- Climate–Data.Org. Dados Climáticos Para Cidades Mundiais. 2020. Available online: https://pt.climate–data.org (accessed on 22 October 2021).
- Mendes, L.C.; Menezes, H.C.; Aparecida, M.; Silva, A.P. Optimization of the roasting of robusta coffee (C. canephora conillon) using acceptability tests and RSM. Food Qual. Prefer. 2001, 12, 153–162. [Google Scholar] [CrossRef]
- Dias, R.C.E.; Campanha, F.G.; Vieira, L.G.E.; Ferreira, L.P.; Pot, D.; Marraccini, P.; Benassi, M.T. Evaluation of kahweol and cafestol in coffee tissues and roasted coffee by a new high–performance liquid chromatography methodology. J. Agric. Food Chem. 2010, 58, 88–93. [Google Scholar] [CrossRef]
- Keidel, A.; von Stetten, D.; Rodrigues, C.; Máguas, C.; Hildebrandt, P. Discrimination of green arabica and robusta coffee beans by Raman spectroscopy. J. Agric. Food Chem. 2010, 58, 11187–11192. [Google Scholar] [CrossRef] [PubMed]
- Rubayiza, A.B.; Meurens, M. Chemical discrimination of arabica and robusta coffees by Fourier Transform Raman Spectroscopy. J. Agric. Food Chem. 2005, 53, 4654–4659. [Google Scholar] [CrossRef] [PubMed]
- Wermelinger, T.; D’Ambrosio, L.; Klopprogge, B.; Yeretzian, C. Quantification of the robusta fraction in a coffee blend via Raman Spectroscopy: Proof of principle. J. Agric. Food Chem. 2011, 59, 9074–9079. [Google Scholar] [CrossRef] [PubMed]
- Sridevi, V.; Giridhar, P.; Ravishankar, G.A. Evaluation of roasting and brewing effect on antinutritional diterpenes–cafestol and kahweol in coffee. Glob. J. Med. Res. 2011, 11, 16–22. [Google Scholar]
- Novaes, F.J.M.; Oigman, S.S.; Souza, R.O.M.A.; Rezende, C.M.; Aquino Neto, F.R. New approaches on the analyses of thermolabile coffee diterpenes by gas chromatography and its relationship with cup quality. Talanta 2015, 139, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.S.G.; Scholz, M.B.S.; Kitzberger, C.S.G.; Benassi, M.T. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Kamm, W.; Dionisi, F.; Fay, L.B.; Hischenhuber, C.; Schmarr, H.G.; Engel, K.H. Rapid and simultaneous analysis of 16–O–methylcafestol and sterols as markers for assessment of green coffee bean authenticity by on–line LC–GC. J. Am. Oil Chem. Soc. 2002, 79, 1109–1113. [Google Scholar] [CrossRef]
- Roos, B.; Van Der Weg, G.; Urgert, R.; Van De Bovenkamp, P.; Charrier, A.; Katan, M.B. Levels of cafestol, kahweol, and related diterpenoids in wild species of the coffee plant Coffea. J. Agric. Food Chem. 1997, 45, 3065–3069. [Google Scholar] [CrossRef] [Green Version]

| Genotypes | |||||
|---|---|---|---|---|---|
| Clone 03 | Clone 05 | Clone 08 | Clone 25 | Clone 66 | |
| Plant size | Medium | Medium | Medium | Medium | Short |
| Main features | High production per branch | Disease resistance | Vigor and high yield | High yield | High yield |
| Maturity | Intermediate 1 | Intermediate/late 2 | Intermediate 1 | Intermediate 1 | Early 3 |
| Fruit size | Medium | Medium | Large | Large | Small |
| Presence in field | 80% | 41% | 89% | 89% | 63% |
| Growing Sites | Genotypes | ||||
|---|---|---|---|---|---|
| Clone 03 | Clone 05 | Clone 08 | Clone 25 | Clone 66 | |
| Alto Alegre dos Parecis | 17.3 aB ± 0.1 | 17.5 bcB ± 0.1 | 18.0 dB ± 0.2 | 39.0 bcA ± 0.3 | 17.6 bB ± 0.1 |
| São Miguel do Guaporé | 0.0 bD ± 0.0 | 0.0 dD ± 0.0 | 36.9 aB ± 1.0 | 38.3 cA ± 1.3 | 17.7 bC ± 0.1 |
| Nova Brasilândia do Oeste | 0.0 bD ± 0.0 | 18.4 abC ± 0.1 | 19.5 cB ± 0.2 | 39.7 bA ± 0.3 | 0.0 cD ± 0.0 |
| Porto Velho | 18.0 aD ± 0.1 | 17.0 cE ± 0.1 | 23.7 bA ± 0.1 | 21.9 dB ± 0.3 | 19.0 aC ± 0.1 |
| Rolim de Moura | 0.0 bC ± 0.0 | 18.4 abA ± 0.1 | 0.0 eC ± 0.0 | 17.4 eB ± 0.1 | 0.0 cC ± 0.0 |
| Alto Paraíso | 17.2 aC ± 0.1 | 18.8 aB ± 0.1 | 18.1 dBC ± 0.1 | 41.6 aA ± 0.5 | 18.8 aB ± 0.1 |
| Growing Sites | Genotypes | ||||
|---|---|---|---|---|---|
| Clone 03 | Clone 05 | Clone 08 | Clone 25 | Clone 66 | |
| Alto Alegre dos Parecis | 242 bB ± 5 | 245 cB ± 2 | 293 cA ± 19 | 243 bcB ± 2 | 306 bA ± 1 |
| São Miguel do Guaporé | 258 abB ± 11 | 243 cBC ± 2 | 268 cB ± 14 | 216 cC ± 13 | 387 aA ± 4 |
| Nova Brasilândia do Oeste | 275 abC ± 0 | 333 bB ± 23 | 448 aA ± 14 | 253 abC ± 9 | 277 bC ± 13 |
| Porto Velho | 251 bC ± 7 | 306 bB ± 9 | 348 bA ± 7 | 281 aBC ± 9 | 96 dD ± 2 |
| Rolim de Moura | 288 aB ± 2 | 258 cB ± 5 | 369 bA ± 32 | 167 dC ± 9 | 136 cC ± 6 |
| Alto Paraíso | 268 abC ± 4 | 457 aA ± 14 | 216 dD ± 7 | 258 abC ± 18 | 374 aB ± 3 |
| Growing Sites | Genotypes | ||||
|---|---|---|---|---|---|
| Clone 03 | Clone 05 | Clone 08 | Clone 25 | Clone 66 | |
| Alto Alegre dos Parecis | 167 fD ± 4 | 233 dB ± 2 | 185 cCD ± 3 | 326 bA ± 1 | 188 bC ± 4 |
| São Miguel do Guaporé | 365 cA ± 4 | 337 bB ± 20 | 263 bD ± 14 | 312 bcC ± 12 | 220 aE ± 1 |
| Nova Brasilândia do Oeste | 330 dA ± 6 | 281 cB ± 8 | 270 bBC ± 2 | 259 dC ± 12 | 181 bD ± 10 |
| Porto Velho | 397 bA ± 5 | 258 cdD ± 0 | 370 aB ± 24 | 298 cC ± 5 | 77 cE ± 1 |
| Rolim de Moura | 433 aA ± 7 | 367 aB ± 2 | 250 bC ± 1 | 155 eD ± 3 | 75 cE ± 5 |
| Alto Paraíso | 291 eB ± 5 | 255 dC ± 0 | 255 bC ± 7 | 354 aA ± 10 | 235 aC ± 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francisco, J.S.; Dias, R.C.E.; Alves, E.A.; Rocha, R.B.; Dalazen, J.R.; Mori, A.L.B.; Benassi, M.d.T. Natural Intervarietal Hybrids of Coffea canephora Have a High Content of Diterpenes. Beverages 2021, 7, 77. https://doi.org/10.3390/beverages7040077
Francisco JS, Dias RCE, Alves EA, Rocha RB, Dalazen JR, Mori ALB, Benassi MdT. Natural Intervarietal Hybrids of Coffea canephora Have a High Content of Diterpenes. Beverages. 2021; 7(4):77. https://doi.org/10.3390/beverages7040077
Chicago/Turabian StyleFrancisco, Julyene Silva, Rafael Carlos Eloy Dias, Enrique Anastácio Alves, Rodrigo Barros Rocha, Janderson Rodrigues Dalazen, André Luiz Buzzo Mori, and Marta de Toledo Benassi. 2021. "Natural Intervarietal Hybrids of Coffea canephora Have a High Content of Diterpenes" Beverages 7, no. 4: 77. https://doi.org/10.3390/beverages7040077
APA StyleFrancisco, J. S., Dias, R. C. E., Alves, E. A., Rocha, R. B., Dalazen, J. R., Mori, A. L. B., & Benassi, M. d. T. (2021). Natural Intervarietal Hybrids of Coffea canephora Have a High Content of Diterpenes. Beverages, 7(4), 77. https://doi.org/10.3390/beverages7040077