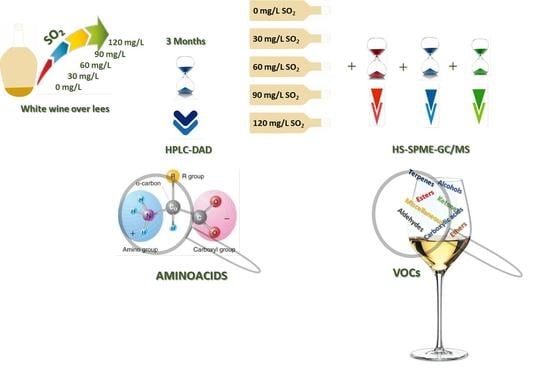
Different SO2 Doses and the Impact on Amino Acid and Volatile Profiles of White Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Wine Samples
2.3. HS-SPME Sampling of Wine Volatiles
2.4. GC/MS Analysis
2.5. Amino Acids Analysis by HPLC-DAD
2.6. Statistical Analysis
3. Results and Discussion
3.1. Volatile Organic Compound
3.1.1. SO2 in Wine Samples
3.1.2. Analysis of Volatile Organic Compounds
3.1.3. Volatile Organic Compounds in Wines
3.1.4. Principal Component Analysis of Volatile Organic Compounds during Bottle Ageing
3.2. Amino Acids
Amino Acids Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernão-Pires, M.J. Vinhos e Aguardentes de Portugal ANUÁRIO 2018; Lisbon, Portugal, 2018. Available online: https://www.ivv.gov.pt/np4/Anuário (accessed on 2 April 2023).
- CVRA. Relatório Anual Gestão e Contas 2019. 2019. Available online: https://www.vinhosdoalentejo.pt/pt/a-cvra/informacao-e-documentos/ (accessed on 2 April 2023).
- Martins, A.A.; Araújo, A.R.; Morgado, A.; Graça, A.; Caetano, N.S.; Mata, T.M. Sustainability Evaluation of a Portuguese “Terroir” Wine. In Chemical Engineering Transactions; AIDIC Servizi S.r.l.: Milano, Italy, 2019; Volume 57, pp. 1945–1950. ISBN 978-88-95608-48-8. ISSN 2283-9216. Available online: https://www.aidic.it/cet/17/57/325.pdf (accessed on 2 April 2023). [CrossRef]
- Alonso González, P.; Parga-Dans, E. Natural Wine: Do Consumers Know What It Is, and How Natural It Really Is? J. Clean. Prod. 2020, 251, 119635. [Google Scholar] [CrossRef]
- Neto, B.; Dias, A.C.; Machado, M. Life Cycle Assessment of the Supply Chain of a Portuguese Wine: From Viticulture to Distribution. Int. J. Life Cycle Assess 2013, 18, 590–602. [Google Scholar] [CrossRef]
- Martins, A.A.; Araújo, A.R.; Graça, A.; Caetano, N.S.; Mata, T.M. Towards Sustainable Wine: Comparison of Two Portuguese Wines. J. Clean. Prod. 2018, 183, 662–676. [Google Scholar] [CrossRef]
- Roberts, A.C.; McWeeny, D.J. The Uses of Sulphur Dioxide in the Food Industry: A Review. Int. J. Food Sci. Technol. 1972, 7, 221–238. [Google Scholar] [CrossRef]
- Giacosa, S.; Río Segade, S.; Cagnasso, E.; Caudana, A.; Rolle, L.; Gerbi, V. SO2 in Wines: Rational Use and Possible Alternatives. In Red Wine Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 309–321. [Google Scholar] [CrossRef]
- Manzocco, L.; Mastrocola, D.; Nicoli, M.C. Chain-Breaking and Oxygen Scavenging Properties of Wine as Affected by Some Technological Procedures. Food Res. Int. 1998, 31, 673–678. [Google Scholar] [CrossRef]
- The European Parliament. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011; The European Parliament: Strasbourg, France, 2011.
- WHO; FAO. Codex Alimentarius Food Labelling Fifth Edition, 5th ed.; FAO: Rome, Italy, 2007.
- International Code of Oenological Practices. Annex Maximum Acceptable Limits; 2015. Organisation Internationale de la Vigne et du Vin, France. Available online: https://www.oiv.int/public/medias/3741/e-code-annex-maximum-acceptable-limits.pdf (accessed on 2 April 2023).
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and Physical Methodologies for the Replacement/ Reduction of Sulfur Dioxide Use during Winemaking: Review of Their Potentialities and Limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the Efficiency of Sulphur Dioxide Replacements in Wine: A Parameter Review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Almeida Santos, C.V.; Gomes da Silva, M.; Cabrita, M.J. Impact of SO2 and Bentonite Addition during Fermentation on Volatile Profile of Two Varietal White Wines. LWT 2020, 133, 109893. [Google Scholar] [CrossRef]
- Morata, A. (Ed.) Red Wine Technology; Elsevier Science: Madrid, Spain, 2019. [Google Scholar]
- Elias, R.J.; Waterhouse, A.L. Controlling the Fenton Reaction in Wine. J. Agric. Food Chem. 2010, 58, 1699. [Google Scholar] [CrossRef]
- McArdle, J.V.; Hoffmann, M.R. Kinetics and Mechanism of the Oxidation of Aquated Sulfur Dioxide by Hydrogen Peroxide at Low PH. J. Phys. Chem. 1983, 87, 5425–5429. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses Andrew. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation Mechanisms Occurring in Wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Reaction of Oxygen and Sulfite in Wine. Am. J. Enol. Vitic. 2016, 67, 13–17. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kilmartin, P.A. Electrochemical Oxidation of Wine Polyphenols in the Presence of Sulfur Dioxide. J. Agric. Food Chem. 2013, 61, 5573–5581. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Barriuso-Esteban, B.; Nieto-Rojo, R.; Aristizábal-López, N. SO2 Protects the Amino Nitrogen Metabolism of Saccharomyces Cerevisiae under Thermal Stress. Microb. Biotechnol. 2012, 5, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Arapitsas, P.; Guella, G.; Mattivi, F. The Impact of SO2 on Wine Flavanols and Indoles in Relation to Wine Style and Age. Sci. Rep. 2018, 8, 858. [Google Scholar] [CrossRef] [Green Version]
- Sartor, S.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Profiling of Free Amino Acids in Sparkling Wines during Over-Lees Aging and Evaluation of Sensory Properties. LWT 2021, 140, 110847. [Google Scholar] [CrossRef]
- Alcaide-Hidalgo, J.M.; Moreno-Arribas, M.V.; Martín-Álvarez, P.J.; Polo, M.C. Influence of Malolactic Fermentation, Postfermentative Treatments and Ageing with Lees on Nitrogen Compounds of Red Wines. Food Chem. 2007, 103, 572–581. [Google Scholar] [CrossRef]
- Marais, J.; Pool, H.J. Effect of Storage Time and Temperature on the Volatile Composition and Quality of Dry White Table Wines. VITIS J. Grapevine Res. 1980, 19, 151–164. [Google Scholar] [CrossRef]
- Karbowiak, T.; Gougeon, R.D.; Alinc, J.-B.; Brachais, L.; Debeaufort, F.; Andrée Voilley, A.A.; Chassagne, D. Wine Oxidation and the Role of Cork. Food Sci. Nutr. 2010, 50, 20–52. [Google Scholar] [CrossRef]
- Organisation Internationale de la Vigne et du Vin—OIV. Compendium of International Methods of Analysis of Wines and Musts (2 Volumes); Organisation Internationale de la Vigne et du Vin—OIV: Paris, France, 2022; Available online: https://www.oiv.int/sites/default/files/publication/2022-10/Compendium%20Methods%20of%20Analysis%20of%20Wine%20and%20Musts%20Vol1%20and%20Vol2.pdf (accessed on 12 April 2023).
- Gómez-Alonso, S.; Hermosín-Gutiérrez, I.; García-Romero, E. Simultaneous HPLC Analysis of Biogenic Amines, Amino Acids, and Ammonium Ion as Aminoenone Derivatives in Wine and Beer Samples. J. Agric. Food Chem. 2007, 55, 608–613. [Google Scholar] [CrossRef]
- Pereira, C.; Mendes, D.; Dias, T.; Garcia, R.; da Silva, M.G.; Cabrita, M.J. Revealing the Yeast Modulation Potential on Amino Acid Composition and Volatile Profile of Arinto White Wines by a Combined Chromatographic-Based Approach. J. Chromatogr. A 2021, 1641, 461991. [Google Scholar] [CrossRef] [PubMed]
- Carrascón, V.; Vallverdú-Queralt, A.; Meudec, E.; Sommerer, N.; Fernandez-Zurbano, P.; Ferreira, V. The Kinetics of Oxygen and SO2 Consumption by Red Wines. What Do They Tell about Oxidation Mechanisms and about Changes in Wine Composition? Food Chem. 2018, 241, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Gambuti, A.; Picariello, L.; Rinaldi, A.; Ugliano, M.; Moio, L. Impact of 5-Year Bottle Aging under Controlled Oxygen Exposure on Sulfur Dioxide and Phenolic Composition of Tannin-Rich Red Wines. Oeno One 2020, 54, 623–636. [Google Scholar] [CrossRef]
- Santos, C.V.A.; da Silva, M.G.; Cabrita, M.J. Volatile Profile of Two Monovarietal White Wines under Different Antioxidant Environments during Storage in Bottle. Ciência Técnica Vitivinícola 2022, 37, 39–59. [Google Scholar] [CrossRef]
- Bianchi, F.; Careri, M.; Mangia, A.; Musci, M. Retention Indices in the Analysis of Food Aroma Volatile Compounds in Temperature-Programmed Gas Chromatography: Database Creation and Evaluation of Precision and Robustness. J. Sep. Sci. 2007, 30, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mattia, C.D.; Piva, A.; Martuscelli, M.; Mastrocola, D.; Sacchetti, G.M. Effect of Sulfites on the in Vitro Antioxidant Activity of Wines. Ital. J. Food Sci. 2015, 27, 505–512. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Oliveira, P.; Baumes, R.L.; Maia, O. Changes in Aromatic Characteristics of Loureiro and Alvarinho Wines during Maturation. J. Food Compos. Anal. 2008, 21, 695–707. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.-T.; Eyres, G.T.; Marriott, P.J. Cumulative Solid Phase Microextraction Sampling for Gas Chromatography-Olfactometry of Shiraz Wine. J. Chromatogr. A 2012, 1255, 221–227. [Google Scholar] [CrossRef]
- Mateus, E.; Barata, R.C.; Zrostlíková, J.; Gomes da Silva, M.G.R.; Paiva, M.R. Characterization of the Volatile Fraction Emitted by Pinus Spp. By One- and Two-Dimensional Chromatographic Techniques with Mass Spectrometric Detection. J. Chromatogr. A 2010, 1217, 1845–1855. [Google Scholar] [CrossRef]
- Lee, S.J.; Noble, A.C. Characterization of Odor-Active Compounds in Californian Chardonnay Wines Using GC-Olfactometry and GC-Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 8036–8044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, Y.; Li, J.; Fan, W.; Jiang, W. Profile of Volatile Compounds in 11 Brandies by Headspace Solid-Phase Microextraction Followed by Gas Chromatography-Mass Spectrometry. J. Food Sci. 2009, 74, C90–C99. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tao, Y.; Wu, Y.; An, R.; Yue, Z. Aroma Compounds and Characteristics of Noble-Rot Wines of Chardonnay Grapes Artificially Botrytized in the Vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Wang, L.; Li, J.M.; Pei, G.R.; Liu, Q.S. Comparison of Volatile Compounds in Two Brandies Using HS-SPME Coupled with GC-O, GC-MS and Sensory Evaluation. S. Afr. J. Enol. Vitic. 2011, 32, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Tao, Y.-S.; Wang, H.; Zhang, L. Impact Odorants of Chardonnay Dry White Wine from Changli County (China). Eur. Food Res. Technol. 2008, 227, 287–292. [Google Scholar] [CrossRef]
- Keyzers, R.A.; Boss, P.K. Changes in the Volatile Compound Production of Fermentations Made from Musts with Increasing Grape Content. J. Agric. Food Chem. 2010, 58, 1153–1164. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Bin, Y.L.; Reeves, M.J.; Duan, C.Q. Influence of Pre-Fermentation Cold Maceration Treatment on Aroma Compounds of Cabernet Sauvignon Wines Fermented in Different Industrial Scale Fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Kong, C.L.; Li, A.H.; Su, J.; Wang, X.C.; Chen, C.Q.; Tao, Y.S. Flavor Modification of Dry Red Wine from Chinese Spine Grape by Mixed Fermentation with Pichia Fermentans and S. Cerevisiae. LWT 2019, 109, 83–92. [Google Scholar] [CrossRef]
- Horvat, I.; Radeka, S.; Plavša, T.; Lukić, I. Bentonite Fining during Fermentation Reduces the Dosage Required and Exhibits Significant Side-Effects on Phenols, Free and Bound Aromas, and Sensory Quality of White Wine. Food Chem. 2019, 285, 305–315. [Google Scholar] [CrossRef]
- Janzantti, N.S.; Monteiro, M. HS–GC–MS–O Analysis and Sensory Acceptance of Passion Fruit during Maturation. J. Food Sci. Technol. 2017, 54, 2594–2601. [Google Scholar] [CrossRef] [Green Version]
- Culleré, L.; Fernández de Simón, B.; Cadahía, E.; Ferreira, V.; Hernández-Orte, P.; Cacho, J. Characterization by Gas Chromatography–Olfactometry of the Most Odor-Active Compounds in Extracts Prepared from Acacia, Chestnut, Cherry, Ash and Oak Woods. LWT—Food Sci. Technol. 2013, 53, 240–248. [Google Scholar] [CrossRef]
- Pereira, C.; Mendes, D.; Martins, N.; Gomes da Silva MGarcia, R.; Cabrita, M.J. A Sustainable Approach Based on the Use of Unripe Grape Frozen Musts to Modulate Wine Characteristics as a Proof of Concept. Beverages 2022, 8, 79–95. [Google Scholar] [CrossRef]
- Cabrita, M.J.; Freitas, A.M.C.; Laureano, O.; Stefano, R.D. Glycosidic Aroma Compounds of Some Portuguese Grape Cultivars. J. Sci. Food Agric. 2006, 86, 922–931. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-la-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.P.; Valentin, D.; Ferreira, V. Fourteen Ethyl Esters of Wine Can Be Replaced by Simpler Ester Vectors without Compromising Quality but at the Expense of Increasing Aroma Concentration. Food Chem. 2020, 307, 125553. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Han, S.-Y.; Li, M.; Sheng, W.-J. Use of Lysozyme and Oligomeric Proanthocyanidin to Reduce Sulfur Dioxide and the Evolution of Volatile Compounds in Italian Riesling Ice Wine During Aging Process. J. Food Process. Preserv. 2017, 41, e12755. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Savastano, M.L.; Benucci, I.; Esti, M. Evolution of Phenolic and Volatile Compounds during Bottle Storage of a White Wine without Added Sulfite. J. Sci. Food Agric. 2020, 100, 775–784. [Google Scholar] [CrossRef]
- Cassino, C.; Tsolakis, C.; Bonello, F.; Gianotti, V.; Osella, D. Wine Evolution during Bottle Aging, Studied by 1H NMR Spectroscopy and Multivariate Statistical Analysis. Food Res. Int. 2019, 116, 566–577. [Google Scholar] [CrossRef]
- Guilloux-Benatier, M.; Chassagne, D. Comparison of Components Released by Fermented or Active Dried Yeasts after Aging on Lees in a Model Wine. J. Agric. Food Chem. 2003, 51, 746–751. [Google Scholar] [CrossRef]





| Compound n° | LRIcal [LRIlit] a | Compound [Common Name] | Most Abundant Ions (m/z) |
|---|---|---|---|
| Esters | |||
| 1 | 892 [863–893] | Ethyl Acetate | 43/61/70 |
| 2 | 957 [955–975] | Ethyl 2-methylpropanoate [Ethyl isobutyrate] | 45/57 |
| 3 | 971 [924–985] | Propyl acetate | 43/61/71 |
| 4 | 1008 [963–1018] | 2-Methylpropyl acetate | 43/56/71 |
| 5 | 1031 [978–1045] | Ethyl butyrate | 71/43/88/73/41 |
| 6 | 1057 [1009–1066] | Ethyl 3-methylbutyrate [Ethyl isovalerate] | 57/41/70/88 |
| 7 | 1062 [1013–1071] | Butyl acetate | 43/56/61 |
| 8 | 1121 [1071–1131] | 3-Methylbutyl acetate [Isoamyl acetate] | 43/55/70 |
| 9 | 1140 [1125–1176] | Pentyl acetate [Amyl acetate] c | 43/55/70 |
| 10 | 1177 b | Methyl 4-methylvalerate d | 57/74/43/87 |
| 11 | 1189 [1143–1175] | Methyl hexanoate c | 74/43/87/55 |
| 12 | 1234 [1198–1244] | Ethyl hexanoate | 88/99/43/70/60 |
| 13 | 1268 [1251–1287] | Hexyl acetate | 43/56/69/61 |
| 14 | 1278 b | 3-Methylbutyl butyrate [Isoamyl butyrate] d | 71/43/105/55 |
| 15 | 1287 b | Ethyl 4-hexenoate isomer | 68/55/41 |
| 16 | 1295 [1283–1305] | Ethyl 3-hexenoate isomer | 41/69/55 |
| 17 | 1301 b | Acetate 4-hexenoate isomer | 67/82/43 |
| 18 | 1312 [1292–1307] | Acetate 3-hexenoate isomer | 67/43/82 |
| 19 | 1329 [1304–1322] | Ethyl heptanoate [Grape oil] | 88/43/70/113/101/60 |
| 20 | 1346 [1327–1353] | Ethyl 2-hexenoate isomer | 55/99/73/41 |
| 21 | 1360 [1304–1322] | Heptyl acetate d | 43/70/56 |
| 22 | 1386 [1351–1391] | Methyl octanoate | 74/87/43/55 |
| 23 | 1430 [1402–1454] | Ethyl octanoate | 88/57/43/127 |
| 24 | 1455 [1455–1472] | 3-Methylbutyl hexanoate [Isoamyl hexanoate] | 70/43/99/55 |
| 25 | 1460 [1429–1489] | Octyl acetate | 43/56/70/83 |
| 26 | 1515 [1508–1538] | Propyl octanoate | 61/145/127/41 |
| 27 | 1531 [1511–1561] | Ethyl nonanoate [Wine ether] | 88/101/70/41/55 |
| 28 | 1534 [1525–1576] | 2-Methylpropyl octanoate [Isobutyl octanoate] | 56/41/127/145 |
| 29 | 1555 [1547–1560] | Octyl formate c | 41/55/69/83 |
| 30 | 1565 [1583–1594] | Hexyl octanoate d | 41/56/127/69/145 |
| 31 | 1591 [1570–1636] | Methyl decanoate | 74/87/43/55 |
| 32 | 1631 [1636–1680] | Ethyl decanoate | 88/70/55 |
| 33 | 1643 [1660–1693] | 3,7-Dimethyl-6-octen-1-yl acetate [Citronellol acetate] c | 41/69/81/55/95 |
| 34 | 1654 [1657–1695] | 3-Methylbutyl octanoate [Isoamyl caprylate] | 70/127/43 |
| 35 | 1661 [1642–1691] | Decyl acetate c | 43/69/55/83 |
| 36 | 1667 [1622–1680] | Diethyl succinate | 101/129/55 |
| 37 | 1686 [1663–1727] | Ethyl 9-decenoate isomer | 55/88/135 |
| 38 | 1731 [1724–1747] | Propyl decanoate | 61/173/41/155 |
| 39 | 1754 [1637–1772] | Ethyl undecanoate d | 41/88/55/70/101 |
| 40 | 1815 [1804–1833] | Methyl dodecanoate [Methyl laurate] | 74/43/127 |
| 41 | 1827 [1782–1852] | 2-Phenylethyl acetate | 104/91/43 |
| 42 | 1838 [1837–1881] | Ethyl dodecanoate [Ethyl laurate] | 88/70/41 |
| 43 | 1859 [1840–1897] | 3-methylbutyl decanoate [Isoamyl decanoate] | 70/43/55 |
| 44 | 1908 [1841] | Ethyl isopentyl succinate | 101/129/55 |
| 45 | 1953 b | Ethyl tridecanoate c | 60/73/88 |
| 46 | NC [2057–2062] | Pentyl laurate [Amyl laurate] c | 43/70/55/143 |
| 47 | NC [2233–2242] | Ethyl hexadecanoate [Ethyl palmitate] | 88/41/157 |
| 48 | NC [2241–2274] | Ethyl tetradecanoate [Ethyl myristate] d | 41/88/70/157 |
| Ethers | |||
| 49 | 1090 b | 1-(1-Ethoxyethoxy)pentane [Acetaldehyde ethyl amyl acetal] | 73/45 |
| 50 | 1756 b | Octyl ether | 57/71/41/83 |
| Ketones | |||
| 51 | 991 [987–991] | Butanedione c | 43/61/86 |
| 52 | 1384 [1386–1387] | 2-Nonanone d | 58/43 |
| Alcohols | |||
| 53 | 930 [927–968] | 2-Propanol | 45/44/43 |
| 54 | 1023 [1012–1032] | 2-Butanol d | 45/59/43 |
| 55 | 1066 [1047–1111] | 2-Methylpropyl alcohol [Isobutanol] | 41/55/73 |
| 56 | 1128 [1102–1175] | 1-Butanol | 43/56/70 |
| 57 | 1197 [1173–1211] | 3-Methylbutan-1-ol [Isopentyl alcohol] | 55/41/70 |
| 58 | 1307 [1313–1357] | 3-Methylpentan-1-ol | 56/96/41 |
| 59 | 1340 [1292–1348] | 1-Hexanol | 56/41/69 |
| 60 | 1375 [1358–1379] | 3-Hexen-1-ol | 67/41/82/55 |
| 61 | 1433 [1428–1457] | 1-Heptanol d | 70/55/41/88 |
| 62 | 1635 [1630–1694] | 1-Nonanol d | 55/41/70/83/97 |
| 63 | 1736 [1720–1794] | 1-Decanol | 41/55/69/83 |
| 64 | 1850 [1760–1799] | 3,7-Dimethyloct-6-en-1-ol [Citronellol] d | 41/67/55/81/95 |
| 65 | 1889 [1846–1870] | Phenylmethyl alcohol | 108/79 |
| 66 | 1918 [1873–1947] | Phenethyl alcohol | 91/65/122 |
| Aldehydes | |||
| 67 | NC [700–744] | Acetaldehyde [Ethanal] | 44/43 |
| 68 | 1407 [1388–1415] | Nonanal c | 41/57/70/82 |
| Carboxylic acids | |||
| 69 | 1918 [1935–1965] | Heptanoic acid | 60/73/41 |
| 70 | NC [2051–2091] | Octanoic acid | 60/70/41/101 |
| 71 | NC [2269–2276] | Decanoic acid | 73/41/129 |
| Miscellaneous | |||
| 72 | 1335 [1309–1363] | Ethyl 2-hydroxypropanoate [Ethyl lactate] | 45/75 |
| 73 | 1366 [1369–1409] | 3-Ethoxypropan-1-ol | 58/45/71 |
| 74 | 1489 [1426–1485] | Furfural | 96/95 |
| 75 | 1541 [1510–1552] | 2-Methylthiolan-3-one d | 60/116 |
| 76 | 1556 [1541–1600] | β-Linalool | 93/71/55/41/121 |
| 77 | 1635 [1618–1621] | Ethyl 2-furylcarboxylate | 95/112 |
| 78 | 1711 [1704–1715] | Terpineol isomer | 59/93/121/136 |
| 79 | 1718 [1698–1755] | 3-(Methylsulfanyl)-1-propanol [Methionol] | 106/57/45/73 |
| 80 | 1783 [1741–1778] | 1,2-Dihydro-1,1,6-trimethylnaphthalene [TDN] c | 157/142 |
| 81 | 1824 [1765–1803] | Methyl 2-hydroxybenzoate [Methyl salicylate] d | 120/92/152/65 |
| 82 | 1867 [1816–1833] | β-Damascenone | 69/121/41 |
| 83 | NC [2042–2057] | Nerolidol isomer | 41/69/93/107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.V.A.; Pereira, C.; Martins, N.; Cabrita, M.J.; Gomes da Silva, M. Different SO2 Doses and the Impact on Amino Acid and Volatile Profiles of White Wines. Beverages 2023, 9, 33. https://doi.org/10.3390/beverages9020033
Santos CVA, Pereira C, Martins N, Cabrita MJ, Gomes da Silva M. Different SO2 Doses and the Impact on Amino Acid and Volatile Profiles of White Wines. Beverages. 2023; 9(2):33. https://doi.org/10.3390/beverages9020033
Chicago/Turabian StyleSantos, Cátia V. Almeida, Catarina Pereira, Nuno Martins, Maria João Cabrita, and Marco Gomes da Silva. 2023. "Different SO2 Doses and the Impact on Amino Acid and Volatile Profiles of White Wines" Beverages 9, no. 2: 33. https://doi.org/10.3390/beverages9020033
APA StyleSantos, C. V. A., Pereira, C., Martins, N., Cabrita, M. J., & Gomes da Silva, M. (2023). Different SO2 Doses and the Impact on Amino Acid and Volatile Profiles of White Wines. Beverages, 9(2), 33. https://doi.org/10.3390/beverages9020033