Canine-Origin Platelet-Rich Fibrin as an Effective Biomaterial for Wound Healing in Domestic Cats: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
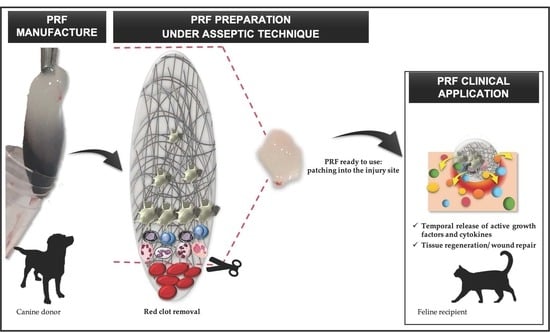
2.1. Production of Xenogenic PRF Clots
2.2. Characterization of the Recipient Feline Population
2.3. PRF Grafting Procedure and Treatment Protocol
2.4. Assessment of the Wound Area Reduction and Statistical Analysis
3. Results
3.1. Assessment of the Wound Area over Time
3.2. Wound Healing Progress during the PRF Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toffler, M.; Toscano, N.; Holtzclaw, D.; Corso, M.D.; Ehrenfest, D.D. Introducing Choukroun’s Platelet Rich Fibrin (PRF) to the Reconstructive Surgery Milieu. J. Implant. Adv. Clin. Dent. 2009, 108, 21–32. [Google Scholar]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Adda, F.; Schoeffler, C.V.A. Une opportunité en paro-implantologie: Le PRF. Implantodontie 2011, 42, 55–62. [Google Scholar]
- Miron, R.J.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Hernandez, M.; Choukroun, J. Platelet-Rich Fibrin and Soft Tissue Wound Healing: A Systematic Review. Tissue Eng.-Part B Rev. 2017, 23, 83–99. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, C.K.L.; Fernandes, B.L.; de Souza, M.A. Autologous Matrix of Platelet-Rich Fibrin in Wound Care Settings: A Systematic Review of Randomized Clinical Trials. J. Funct. Biomater. 2020, 11, 31. [Google Scholar] [CrossRef]
- Cortese, A.; Pantaleo, G.; Borri, A.; Caggiano, M.; Amato, M. Platelet-rich fibrin (PRF) in implant dentistry in combination with new bone regenerative technique in elderly patients. Int. J. Surg. Case Rep. 2016, 28, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Mohammed Al Ashmawy, M.M.; Mohamed Ali, H.E.D.; Baiomy Abdullah Baiomy, A.A. Effect of Platelet-Rich Fibrin on The Regeneration Capacity of Bone Marrow Aspirate in Alveolar Cleft Grafting (Clinical and Radiographic Study). Dentistry 2017, 7, 2161-1122. [Google Scholar] [CrossRef]
- Kazemi, D.; Fakhrjou, A. Leukocyte and Platelet Rich Plasma (L-PRP) Versus Leukocyte and Platelet Rich Fibrin (L-PRF) For Articular Cartilage Repair of the Knee: A Comparative Evaluation in an Animal Model. Iran. Red Crescent Med. J. 2015, 17, e19594. [Google Scholar] [CrossRef] [Green Version]
- Martínez, C.E.; Smith, P.C.; Palma Alvarado, V.A. The influence of platelet-derived products on angiogenesis and tissue repair: A concise update. Front. Physiol. 2015, 6, 290. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Aristizabal, R.F.; López, C.; Álvarez, M.E.; Giraldo, C.; Prades, M.; Carmona, J.U. Long-term cytokine and growth factor release from equine platelet-rich fibrin clots obtained with two different centrifugation protocols. Cytokine 2017, 97, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich Concentrates Differentially Release Growth Factors and Induce Cell Migration In Vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preeja, C.; Arun, S. Platelet-rich fibrin: Its role in periodontal regeneration. Saudi J. Dent. Res. 2014, 5, 117–122. [Google Scholar] [CrossRef] [Green Version]
- Masuki, H.; Okudera, T.; Watanabe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant. Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [Green Version]
- Naik, B.; Karunakar, P.; Jayadev, M.; Marshal, V.R. Role of Platelet rich fibrin in wound healing: A critical review. J. Conserv. Dent. 2013, 16, 284. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.S.; Barros, L.C.; Saraiva, V.; Gomez-Florit, M.; Babo, P.S.; Dias, I.R.; Reis, R.L.; Carvalho, P.P.; Gomes, M.E. Bioengineered surgical repair of a chronic oronasal fistula in a cat using autologous platelet-rich fibrin and bone marrow with a tailored 3D printed implant. J. Feline Med. Surg. 2018, 20, 835–843. [Google Scholar] [CrossRef]
- Soares, C.S.; Babo, P.S.; Reis, R.L.; Carvalho, P.P.; Gomes, M.E. Platelet-Derived Products in Veterinary Medicine: A New Trend or an Effective Therapy? Trends Biotechnol. 2021, 39, 225–243. [Google Scholar] [CrossRef]
- Soares, C.S.; Babo, P.S.; Faria, S.; Pires, M.A.; Carvalho, P.P. Standardized Platelet-Rich Fibrin (PRF) from canine and feline origin: An analysis on its secretome pattern and architectural structure. Cytokine 2021, 148, 155695. [Google Scholar] [CrossRef]
- Gemignani, F.; Perazzi, A.; Iacopetti, I. Use of canine sourced platelet-rich plasma in a feline contaminated cutaneous wound. Can. Vet. J. 2017, 58, 141–144. [Google Scholar] [PubMed]
- Pietro, S.D.; Chiara, C.; Francesca, A.; Rosario, P. Platelet Rich Plasma Eye Drops: Preparation, Storage and Clinical Use in Dogs and Cats. Preliminary Results. Arch. Vet. Sci. Technol. 2017, 1, 1–4. [Google Scholar]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Dohan Ehrenfest, M.D.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2017, 29, 468–475. [Google Scholar] [CrossRef]
- Shreyas, N.S.; Agrwal, S.; Agrwal, C.; Karki, S.; Khare, S. Efficacy of autologous platelet-rich fibrin in chronic cutaneous ulcer: A randomized controlled trial. Indian J. Clin. Exp. Dermatol. 2019, 3, 172–181. [Google Scholar]
- Somani, A.; Rai, R. Comparison of efficacy of autologous platelet-rich fibrin versus saline dressing in chronic venous leg ulcers: A randomised controlled trial. J. Cutan. Aesthet. Surg. 2017, 10, 8–12. [Google Scholar] [PubMed]
- Vaheb, M.; Karrabi, M.; Khajeh, M.; Asadi, A.; Shahrestanaki, E.; Sahebkar, M. Evaluation of the Effect of Platelet-Rich Fibrin on Wound Healing at Split-Thickness Skin Graft Donor Sites: A Randomized, Placebo-Controlled, Triple-Blind Study. Int. J. Low. Extrem. Wounds 2020, 20, 29–36. [Google Scholar] [CrossRef]
- Calis, H.T.; Sütbeyaz, S.T.; Güler, E.; Halici, C.; Sayan, H.; Koc, A.; Konk, M.; Yazicioglu, J.A. Efficacy of Intra-Articular Autologous Platelet Rich Plasma Application in Knee Osteoarthritis. Arch. Rheumatol. 2015, 30, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Filardo, G.; Kon, E.; Roffi, A.; Di Matteo, B.; Merli, M.L.; Marcacci, M. Platelet-rich plasma: Why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg. Sport Traumatol. Arthrosc. 2015, 23, 2459–2474. [Google Scholar] [CrossRef] [Green Version]
- Gillitzer, R.; Goebeler, M. Chemokines in cutaneous wound healing. J. Leukoc. Biol. 2001, 69, 513–521. [Google Scholar] [PubMed]
- Gordon, J.; Ley, S.C.; Melamed, M.D.; English, L.S.; Hughes-Jones, N.C. Immortalized B lymphocytes produce B-cell growth factor. Nature 1984, 310, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The structure and biological features of fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Ozer, K.; Colak, O. Leucocyte- and platelet-rich fibrin as a rescue therapy for small-to-medium-sized complex wounds of the lower extremities. Burn. Trauma 2019, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Diss, A.; Mouhyi, J.; Charrier, J.B. Three-Dimensional Architecture and Cell Composition of a Choukroun’s Platelet-Rich Fibrin Clot and Membrane. J. Periodontol. 2010, 81, 546–555. [Google Scholar] [CrossRef]
- Borie, E.; Oliví, D.G.; Orsi, I.A.; Garlet, K.; Weber, B.; Beltrán, V.; Fuentes, R. Platelet-rich fibrin application in dentistry: A literature review. Int. J. Clin. Exp. Med. 2015, 8, 7922–7929. [Google Scholar]
- Cieślik-Bielecka, A.; Bold, T.; Ziółkowski, G.; Pierchała, M.; Królikowska, A.; Reichert, P. Antibacterial Activity of Leukocyte- and Platelet-Rich Plasma: AnIn VitroStudy. BioMed Res. Int. 2018, 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsujino, T.; Takahashi, A.; Yamaguchi, S.; Watanabe, T.; Isobe, K.; Kitamura, Y.; Tanaka, T.; Nakata, K.; Kawase, T. Evidence for Contamination of Silica Microparticles in Advanced Platelet-Rich Fibrin Matrices Prepared Using Silica-Coated Plastic Tubes. Biomedicines 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peck, M.T.; Hiss, D.; Stephen, L.; Satti, A.; Majeed, A. Platelet-Rich Fibrin (PRF)—The effect of storage time on platelet concentration. South Afr. Dent. J. 2015, 70, 448–451. [Google Scholar]
- Bottegoni, C.; Dei Giudici, L.; Salvemini, S.; Chiurazzi, E.; Bencivenga, R.; Gigante, A. Homologous platelet-rich plasma for the treatment of knee osteoarthritis in selected elderly patients: An open-label, uncontrolled, pilot study. Ther. Adv. Musculoskelet. Dis. 2016, 8, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.M. Component Therapy. In Manual of Veterinary Transfusion Medicine and Blood Banking; Kenichiro Yagi, M.K.H., Ed.; Wiley-Blackwell: Oxford, UK, 2016; pp. 13–28. [Google Scholar]
- European Directorate for the Quality, of Medicines & HealthCare (EDQM) C of E. Guide to the Preparation, Use and Quality Assurance of Blood Components. Available online: http://www.centronazionalesangue.it/sites/default/files/guida_edqm_16_edizione.pdf (accessed on 30 March 2021).
- Burnouf, T.; Chou, M.-L.; Wu, Y.-W.; Su, C.-Y.; Lee, L.-W. Antimicrobial activity of platelet (PLT)-poor plasma, PLT-rich plasma, PLT gel, and solvent/detergent-treated PLT lysate biomaterials against wound bacteria. Transfusion 2013, 53, 138–146. [Google Scholar] [CrossRef]
- Feng, M.; Wang, Y.; Zhang, P.; Zhao, Q.; Yu, S.; Shen, K.; Miron, R.J.; Zhang, Y. Antibacterial effects of platelet-rich fibrin produced by horizontal centrifugation. Int. J. Oral Sci. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Hamed, M.A.; Abouelnasr, K.S.; El-Adl, M.; Abo Elfadl, E.A.; Farag, A.; Lashen, S. Effectiveness of Allogeneic Platelet-Rich Fibrin on Second-Intention Wound Healing of Experimental Skin Defect in Distal Limb in Donkeys (Equus asinus). J. Equine Vet. Sci. 2019, 73, 131–138. [Google Scholar] [CrossRef]







| Canine Donor | Age (years) | Sex, Reproductive Status | Breed |
|---|---|---|---|
| A | 7 | M, neutered | Labrador retriever |
| B | 2 | F, neutered | Beagle |
| C | 2 | M, intact | Boerboel |
| D | 8 | M, neutered | American Pit Bull Terrier |
| Case | Age | Sex | Weight (kg) | Origin of the Lesion | Lesion Localization | Co-Mobilities |
|---|---|---|---|---|---|---|
| 1 | 9 Mo | Male, neutered | 2.600 | Surgical wound dehiscence, after the repair of a metaphysis fracture | Left knee, anterior region | Polytraumatized patient (bone fracture, pancreatitis, and liver lesions) resulted from a 4th-floor fall. Submitted to orthopedic surgery after medical stabilization. |
| 2 | 2 Yr | Female, neutered | 3.100 | Abscess due to a dog attack | Lateral region of proximal right hind limb | Fracture of the femur, a consequence of a dog attack, ventral abdominal wall laceration, hepatic lipidosis during the recovery period. |
| 3 | 10 Yr | Male, neutered | 4.480 | Abscess resulted from a catfight | Left facial region | FIV +; mild anemia (30% hematocrit). |
| 4 | 4 Yr | Male, intact | 3.830 | Extensive laceration with 24 h (broken glass window) | Distal hind limb, tarsus region | FIV +; moderate anemia (24% hematocrit); Bartonella sp. +. |
| Case | Time Point Day | Wound Area (cm2) | PRFs Applied | Donor a | Wound/Study Duration | Clinical Evaluation | Adverse Effects | Considerations |
|---|---|---|---|---|---|---|---|---|
| 1 | D1 | 5.47 | 2 | B | 8 days b | Pale color and intense fibrin deposition. Moderate exudate and thickened borders. | - | Hospitalized feline: mechanical revitalization of the lesion bed. |
| D4 | NP | 2 | A | Pink granulation tissue Moderate exudate and thickening of the borders. | Not observed | Outpatient treatment. | ||
| D8 | 3.73 | 0 End of the study | NP | Exuberant granulation tissue and vascularization signs. Decreased exudate production. Epithelization. | Not observed | Outpatient treatment. Did not complete the study (hind limb amputation). | ||
| 2 | D1 | 2.26 | 1 | A | 11 days | Presence of infection (yellow purulent exudate). Depth wound (1.1 cm), with a muscular laceration. Erythema and oedema. | - | Hospitalized feline: surgical debridement, under surgery. |
| D3 | 0.62 | 1 | A | Less tumefaction. Vestigial exudate. Normal appearance of the skin. | Not observed | Hospitalized feline (hepatic lipidosis secondary to trauma). | ||
| D6 | 0.25 | 0 Only closed bandage | NP | No exudate. Dry adherent crust. No tumefaction. Hair growing. | Not observed | The patient was discharged (hepatic recovery). | ||
| D11 | 0.00 | End of the study | NP | Crust detachment. Contracted skin, with no subcutaneous fibrosis. | Not observed | Reported by the owner: outpatient treatment. | ||
| 3 | D1 | 3.42 | 1 | C | 12 days | Presence of infection (green purulent exudate, with odor). Erythema and oedema. | Not observed | Hospitalized feline (under sedation). |
| D3 | 2.28 | 1 | C | Less tumefaction. Moderate exudate and mild erythema. | Not observed | Hospitalized feline: medical drainage, under sedation. | ||
| D8 | 0.08 | 0 Only closed bandage | NP | Dry lesion. Evident contraction and crust formation. Hair growing. | Not observed | Hospitalized feline. | ||
| D12 | 0.00 | End of the study | NP | Normal skin. No subcutaneous fibrosis. | Not observed | Outpatient treatment. | ||
| 4 | D1 | 7.01 | 4 | D | 42 days | Presence of infection (purulent yellow exudate, with odor). Visualization of the ligaments/tendons. Wedge detachment. Marked edema. | Not observed | Hospitalized feline: medical debridement, under sedation. |
| D3 | 5.68 | 3 | D | Wound contraction and moderate exudate. Pale tissue and thickening of borders | Not observed | Hospitalized feline (under sedation). | ||
| D5 | 4.93 | 2 | D | Pink color: vascularization signs. Border with mild erythema | Not observed Uncooperative patient | Outpatient treatment. | ||
| D11 | 4.85 | 2 | A | Moderate granulation tissue: vascularization signs. Mild fibrin deposition. | Not observed Cooperative patient | Outpatient treatment. Applied PRFs from a new donor. | ||
| D 17 | NP | 0 Only closed bandage | NP | Exophytic granulation tissue (PRF treatment was suspended). Mild exudate. Hair growing (local trichotomy performed). | Not observed Cooperative patient | Outpatient treatment. | ||
| D24 | 2.08 | 0 Only closed bandage | NP | Wound contraction. Dark granulation tissue and mild exudate. Epithelialization. | Not observed Cooperative patient | Outpatient treatment. | ||
| D29 | 1.05 | 0 Only closed bandage | NP | Wound contraction. Dry lesion Epithelialization. | Not observed | Outpatient treatment. | ||
| D42 | 0.00 | End of the study | NP | Normal skin. No subcutaneous fibrosis. Skin contraction. | Not observed | Outpatient treatment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, C.S.; Dias, I.R.; Pires, M.A.; Carvalho, P.P. Canine-Origin Platelet-Rich Fibrin as an Effective Biomaterial for Wound Healing in Domestic Cats: A Preliminary Study. Vet. Sci. 2021, 8, 213. https://doi.org/10.3390/vetsci8100213
Soares CS, Dias IR, Pires MA, Carvalho PP. Canine-Origin Platelet-Rich Fibrin as an Effective Biomaterial for Wound Healing in Domestic Cats: A Preliminary Study. Veterinary Sciences. 2021; 8(10):213. https://doi.org/10.3390/vetsci8100213
Chicago/Turabian StyleSoares, Carla S., Isabel R. Dias, Maria A. Pires, and Pedro P. Carvalho. 2021. "Canine-Origin Platelet-Rich Fibrin as an Effective Biomaterial for Wound Healing in Domestic Cats: A Preliminary Study" Veterinary Sciences 8, no. 10: 213. https://doi.org/10.3390/vetsci8100213
APA StyleSoares, C. S., Dias, I. R., Pires, M. A., & Carvalho, P. P. (2021). Canine-Origin Platelet-Rich Fibrin as an Effective Biomaterial for Wound Healing in Domestic Cats: A Preliminary Study. Veterinary Sciences, 8(10), 213. https://doi.org/10.3390/vetsci8100213