News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation
Abstract
:1. Introduction
2. Methods
2.1. Procedural Management
2.2. Ablation Procedure
3. Statistical Analysis
4. Results
4.1. Baseline Characteristics
4.2. Procedural Characteristics
4.3. Acute Procedural Outcome
4.4. Cryoablation Freeze Temperature
4.5. Procedure-Related Complications
5. Discussion
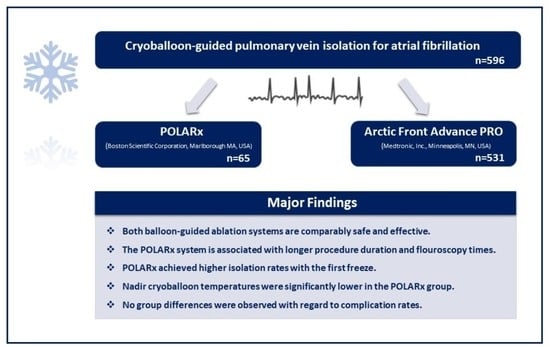
- Both balloon-guided ablation systems are comparably safe and effective for acute single-shot PVI.
- AF ablation utilizing the POLARx system is associated with longer procedure duration and fluoroscopy times. POLARx achieved higher isolation rates with the first freeze.
- Nadir cryoballoon temperatures were significantly lower in the POLARx group.
- No group differences were observed with regard to complication rates.
- Long-term data and assessment of lesion formation are warranted.
5.1. Safety First
5.2. Same but Different
5.3. Things to Consider Using a New Ablation Device
5.4. Acute Procedural Success
5.5. Minimal Freezing Temperature
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021, 42, 373–498. [Google Scholar]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018, 20, e1–e160. [Google Scholar] [CrossRef] [PubMed]
- Asad, Z.U.A.; Yousif, A.; Khan, M.S.; Al-Khatib, S.M.; Stavrakis, S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Circ. Arrhythm Electrophysiol. 2019, 12, e007414. [Google Scholar] [CrossRef]
- Unland, R.; Bergau, L.; El Hamriti, M.; Guckel, D.; Piran, M.; Fink, T.; Sciacca, V.; Köerperich, H.; Chmelevsky, M.; Imnadze, G.; et al. Find Me If You Can: First Clinical Experience Using the Novel CARTOFINDER Algorithm in a Routine Workflow for Atrial Fibrillation Ablation. J. Clin. Med. 2021, 10, 2979. [Google Scholar] [CrossRef] [PubMed]
- Kuck, K.H.; Brugada, J.; Furnkranz, A.; Metzner, A.; Ouyang, F.; Chun, K.R.; Elvan, A.; Arentz, T.; Bestehorn, K.; Pocock, S.J.; et al. FIRE AND ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N. Engl. J. Med. 2016, 374, 2235–2245. [Google Scholar] [CrossRef] [PubMed]
- Luik, A.; Radzewitz, A.; Kieser, M.; Walter, M.; Bramlage, P.; Hormann, P.; Schmidt, K.; Horn, N.; Brinkmeier-Theofanopoulou, M.; Kunzmann, K.; et al. Cryoballoon versus open irrigated radiofrequency ablation in patients with paroxysmal atrial fibrillation: The prospective, randomized, controlled, noninferiority FreezeAF study. Circulation 2015, 132, 1311–1319. [Google Scholar] [CrossRef]
- Providencia, R.; Defaye, P.; Lambiase, P.D.; Pavin, D.; Cebron, J.P.; Halimi, F.; Anselme, F.; Srinivasan, N.; Albenque, J.P.; Boveda, S.; et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: Is cryoablation more reproducible? Europace 2017, 19, 48–57. [Google Scholar] [CrossRef]
- Boveda, S.; Metzner, A.; Nguyen, D.Q.; Chun, K.R.J.; Goehl, K.; Noelker, G.; Deharo, J.C.; Andrikopoulos, G.; Dahme, T.; Lellouche, N.; et al. Single-Procedure Outcomes and Quality-of-Life Improvement 12 Months Post-Cryoballoon Ablation in Persistent Atrial Fibrillation: Results From the Multicenter CRYO4PERSISTENT AF Trial. JACC Clin. Electrophysiol. 2018, 4, 1440–1447. [Google Scholar] [CrossRef]
- Su, W.W.; Reddy, V.Y.; Bhasin, K.; Champagne, J.; Sangrigoli, R.M.; Braegelmann, K.M.; Kueffer, F.J.; Novak, P.; Gupta, S.K.; Yamane, T.; et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: Results from the multicenter STOP Persistent AF trial. Heart Rhythm. 2020, 17, 1841–1847. [Google Scholar] [CrossRef]
- Guckel, D.; Schmidt, A.; Gutleben, K.J.; Körber, B.; Fischbach, T.; Horstkotte, D.; Sommer, P.; Nölker, G. Pulmonary vein isolation and beyond: Predictive value of vagal reactions in second-generation cryoballoon ablation for the outcome of persistent atrial fibrillation. Heart Rhythm. 2020, 17, 600–606. [Google Scholar] [CrossRef]
- Bergau, L.; El Hamriti, M.; Rubarth, K.; Dagher, L.; Molatta, S.; Braun, M.; Khalaph, M.; Imnadze, G.; Nölker, G.; Nowak, C.P.; et al. Cool enough? Lessons learned from cryoballoon-guided catheter ablation for atrial fibrillation in young adults. J. Cardiovasc. Electrophysiol. 2020, 31, 2857–2864. [Google Scholar] [CrossRef]
- Sohns, C.; Marrouche, N.F.; Costard-Jäckle, A.; Sossalla, S.; Bergau, L.; Schramm, R.; Fuchs, U.; Omran, H.; Rubarth, K.; Dumitrescu, D.; et al. Catheter ablation for atrial fibrillation in patients with end-stage heart failure and eligibility for heart transplantation. ESC Heart Fail. 2021, 8, 1666–1674. [Google Scholar] [CrossRef]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Guckel, D.; Isgandarova, K.; Bergau, L.; Piran, M.; El Hamriti, M.; Imnadze, G.; Braun, M.; Khalaph, M.; Fink, T.; Sciacca, V.; et al. The Effect of Diabetes Mellitus on the Recurrence of Atrial Fibrillation after Ablation. J. Clin. Med. 2021, 10, 4863. [Google Scholar] [CrossRef] [PubMed]
- Creta, A.; Kanthasamy, V.; Schilling, R.J.; Rosengarten, J.; Khan, F.; Honarbakhsh, S.; Earley, M.J.; Hunter, R.J.; Finlay, M. First experience of POLARx versus Arctic Front Advance: An early technology comparison. J. Cardiovasc. Electrophysiol. 2021, 32, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Garg, L.; Santangeli, P. Arctic Front versus POLARx cryoballoon: Is there a winner? J. Cardiovasc. Electrophysiol. 2021, 32, 595–596. [Google Scholar] [CrossRef] [PubMed]
- Tilz, R.R.; Meyer-Saraei, R.; Eitel, C.; Fink, T.; Sciacca, V.; Lopez, L.D.; Kirstein, B.; Schlüter, M.; Vogler, J.; Kuck, K.H.; et al. Novel Cryoballoon Ablation System for Single Shot Pulmonary Vein Isolation—The Prospective ICE-AGE-X Study. Circ. J. 2021, 85, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Su, W.W. A second cryoballoon system-New and improved? J. Cardiovasc. Electrophysiol. 2021, 32, 931–932. [Google Scholar] [CrossRef]
- Yap, S.C.; Anic, A.; Breskovic, T.; Haas, A.; Bhagwandien, R.E.; Jurisic, Z.; Szili-Torok, T.; Luik, A.; Yap, S.C. Comparison of procedural efficacy and biophysical parameters between two competing cryoballoon technologies for pulmonary vein isolation: Insights from an initial multicenter experience. J. Cardiovasc. Electrophysiol. 2021, 32, 580–587. [Google Scholar] [CrossRef]
- Assaf, A.; Bhagwandien, R.; Szili-Torok, T.; Yap, S.C.; Assaf, A. Comparison of procedural efficacy, balloon nadir temperature, and incidence of phrenic nerve palsy between two cryoballoon technologies for pulmonary vein isolation: A systematic review and meta-analysis. J. Cardiovasc. Electrophysiol. 2021, 32, 2424–2431. [Google Scholar] [CrossRef]
- Kochi, A.N.; Moltrasio, M.; Tundo, F.; Riva, S.; Ascione, C.; Dessanai, M.A.; Pizzamiglio, F.; Vettor, G.; Cellucci, S.; Gasperetti, A.; et al. Cryoballoon atrial fibrillation ablation: Single-center safety and efficacy data using a novel cryoballoon technology compared to a historical balloon platform. J. Cardiovasc. Electrophysiol. 2021, 32, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.R.J.; Perrotta, L.; Bordignon, S.; Khalil, J.; Dugo, D.; Konstantinou, A.; Fürnkranz, A.; Schmidt, B. Complications in Catheter Ablation of Atrial Fibrillation in 3,000 Consecutive Procedures: Balloon Versus Radiofrequency Current Ablation. JACC Clin. Electrophysiol. 2017, 3, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Anic, A.; Lever, N.; Martin, A.; Breskovic, T.; Sulkin, M.S.; Duffy, E.; I Saliba, W.; Niebauer, M.J.; Wazni, O.M.; Varma, N. Acute safety, efficacy, and advantages of a novel cryoballoon ablation system for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: Initial clinical experience. Europace 2021, 23, 1237–1243. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | POLARx | AFA |
|---|---|---|
| Sheath diameter (F) | 12.7 | 12 |
| Sheath outer diameter (F) | 15.9 | 15 |
| Radiopaque marker proximal to the tip (mm) | 2.5 | 5 |
| Balloon size (mm) | 28 | 28 |
| Balloon shaft diameter (F) | 10.5 | |
| Balloon tip length (mm) | 5 or 12 | 8 |
| N2O injection | 8-hole coil | 8-hole coil |
| N2O fluid flow during freeze (sccm) | 7800 | 7200 |
| Pressure during freeze (psi) | <525 constant | 530–600 |
| Location of injection coil from pole of balloon (mm) | 2.5 | 3.5 |
| Location of TC from coil (mm) | 18 | 15 |
| Location of gas outflow proximal of TC (mm) | 5 | 10 |
| Phrenic nerve palsy control | DMS (integrated/quantitative) | CMAP (not integrated/not quantitative) |
| Console register procedural data | yes | no |
| Console operation autonomically | yes | no |
| Characteristics | POLARx (n = 65) | AFA (n = 531) | p-Value |
|---|---|---|---|
| Age (years) | 65.0 ± 11.6 | 63.0 ± 27.9 | 0.221 |
| Gender, female | 20 (31%) | 132 (25%) | 0.067 |
| BMI (kg/m2) | 30.6 ± 8.8 | 28.6 ± 5.7 | 0.060 |
| LVEF (%) | 52.8 ± 7.7 | 53.6 ± 4.1 | 0.137 |
| Cardiomyopathy | 8 (12%) | 43 (8%) | 0.087 |
| Hypertension | 37 (57%) | 220 (41%) | <0.001 * |
| Diabetes mellitus | 7 (11%) | 80 (15%) | 0.104 |
| Beta blocker | 54 (83%) | 421 (79%) | 0.101 |
| AADs | 5 (8%) | 40 (8%) | 0.173 |
| PAF | 43 (66%) | 281 (53%) | 0.018 * |
| Early recurrence | 7 (11%) | 51 (10%) | 0.148 |
| Characteristics | POLARx (n = 65) | AFA (n = 531) | p-Value |
|---|---|---|---|
| Total procedure time (min) | 113.3 ± 23.2 [96.0, 130.0] | 100.9 ± 21.3 [85.0, 114.0] | <0.001 * |
| Total fluoroscopy time (min) | 10.5 ± 5.9 [6.7, 12.5] | 4.8 ± 3.6 [2.5, 6.2] | <0.001 * |
| Contrast agent (mL) | 38.1 ± 13.8 [30.0, 46.5] | 42.9 ± 16.5 [30.0, 60.0] | 0.075 |
| Cumulative radiation dose (cGycm2) | 432.8 ± 639.4 [116.0, 442.7] | 519.9 ± 363.5 [242.0, 701.0] | 0.300 |
| POLARx (n = 65) | AFA (n = 531) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LSPV (n = 65) | LIPV (n = 65) | LCV (n = 0) | RIPV (n = 65) | RSPV (n = 65) | LSPV (n = 519) | LIPV (n = 519) | LCV (n = 12) | RIPV (n = 531) | RSPV (n = 531) | ||
| Isolation of PV (%) | 100 | 100 | - | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 1.000 |
| Isolation with 1st freeze (%) | 66 | 86 | - | 78 | 67 | 64 | 50 | 8 | 72 | 48 | 0.027 * |
| Isolation with 2nd freeze (%) | 32 | 14 | - | 20 | 30 | 33 | 48 | 42 | 23 | 46 | 0.038 * |
| Isolation with 3rd freeze or more (%) | 2 | 0 | - | 2 | 3 | 3 | 2 | 50 | 5 | 6 | 0.205 |
| Characteristics | POLARx (n = 65) | AFA (n = 531) | p-Value |
|---|---|---|---|
| LSPV | |||
| Minimal temperature (C°) | −58.2 ± 5.3 [−61.0, −55.0] | −46.0 ± 5.8 [−49.0, −43.0] | <0.001 * |
| LIPV | |||
| Minimal temperature (C°) | −56.9 ± 5.6 (−60.0, −53.0] | −41.3 ± 4.7 [−44.8, −39.0] | <0.001 * |
| LCV | |||
| Minimal temperature (C°) | N/A | −38.0 ± 14.2 [−43.0, −27.0] | N/A |
| RIPV | |||
| Minimal temperature (C°) | −58.8 ± 6.5 [−63.0, −54.8] | −47.4 ± 7.1 [−52.0, −42.3] | <0.001 * |
| RSPV | |||
| Minimal temperature (C°) | −56.9 ± 7.6 [−62.0, −53.0] | −45.9 ± 6.7 [−51.0, −41.0] | <0.001 * |
| Characteristics | POLARx (n = 65) | AFA (n = 531) | p-Value |
|---|---|---|---|
| Life threatening complications | |||
| esophageal perforation/fistula | 0 (0%) | 0 (0%) | N/A |
| Periprocedural thromboembolic event | 0 (0%) | 1 (<1%) | 0.902 |
| Cardiac tamponade | 1 (2%) | 2 (<1%) | 0.065 |
| Severe complications | |||
| Persistent phrenic nerve palsy | 0 (0%) | 2 (<1%) | 0.813 |
| Vascular complications | 0 (0%) | 2 (<1%) | 0.813 |
| Moderate or minor complications | |||
| Various | 0 (0%) | 0 (0%) | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guckel, D.; Lucas, P.; Isgandarova, K.; El Hamriti, M.; Bergau, L.; Fink, T.; Sciacca, V.; Imnadze, G.; Braun, M.; Khalaph, M.; et al. News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation. J. Cardiovasc. Dev. Dis. 2022, 9, 16. https://doi.org/10.3390/jcdd9010016
Guckel D, Lucas P, Isgandarova K, El Hamriti M, Bergau L, Fink T, Sciacca V, Imnadze G, Braun M, Khalaph M, et al. News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation. Journal of Cardiovascular Development and Disease. 2022; 9(1):16. https://doi.org/10.3390/jcdd9010016
Chicago/Turabian StyleGuckel, Denise, Philipp Lucas, Khuraman Isgandarova, Mustapha El Hamriti, Leonard Bergau, Thomas Fink, Vanessa Sciacca, Guram Imnadze, Martin Braun, Moneeb Khalaph, and et al. 2022. "News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation" Journal of Cardiovascular Development and Disease 9, no. 1: 16. https://doi.org/10.3390/jcdd9010016
APA StyleGuckel, D., Lucas, P., Isgandarova, K., El Hamriti, M., Bergau, L., Fink, T., Sciacca, V., Imnadze, G., Braun, M., Khalaph, M., Nölker, G., Sommer, P., & Sohns, C. (2022). News from the Cold Chamber: Clinical Experiences of POLARx versus Arctic Front Advance for Single-Shot Pulmonary Vein Isolation. Journal of Cardiovascular Development and Disease, 9(1), 16. https://doi.org/10.3390/jcdd9010016