Maternal Glycemic Spectrum and Adverse Pregnancy and Perinatal Outcomes in a Multiracial US Cohort
Abstract
:1. Introduction
2. Methods
2.1. Data Source, Study Design, and Study Population
2.2. Assessment of Primary Outcomes
2.3. Assessment of Secondary Outcomes
2.4. Glycemic Status
2.5. Assessment of Covariates
2.6. Statistical Analysis
3. Results
3.1. Sociodemographic and Cardiovascular Risk Factors by Maternal Glycemic Subgroups
3.2. Maternal Glycemia and Adverse Pregnancy Outcomes
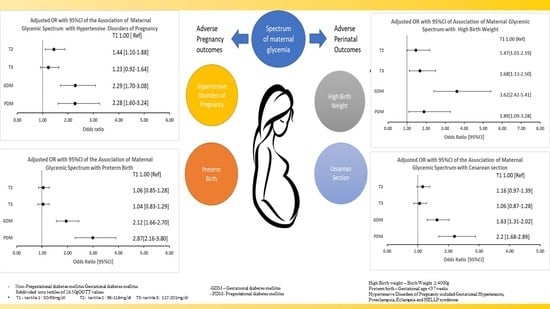
3.2.1. Hypertensive Disorders in Pregnancy
3.2.2. Preterm Birth
3.3. Maternal Glycemia and Perinatal Outcomes
3.3.1. Birth Weight
3.3.2. Cesarean Section
3.4. Maternal Glycemia and Adverse Pregnancy Outcomes by Race-Ethnicity
3.4.1. Hypertensive Disorders of Pregnancy
3.4.2. Preterm Birth
3.5. Maternal Glycemia and Perinatal Outcomes by Race-Ethnicity
3.5.1. Birth Weight
3.5.2. Cesarean Section
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APOs | adverse pregnancy outcomes |
| aOR | adjusted odds ratio |
| BMI | body mass index |
| CS | cesarean section |
| GDM | gestational diabetes |
| HBW | high birth weight |
| HDP | hypertensive disorders of pregnancy |
| NHB | non-Hispanic Black |
| OGTT | oral glucose tolerance test |
| PDM | pregestational diabetes |
| PTB | preterm birth |
References
- Grandi, S.M.; Filion, K.B.; Yoon, S.; Ayele, H.T.; Doyle, C.M.; Hutcheon, J.A.; Smith, G.; Gore, G.C.; Ray, J.G.; Nerenberg, K.; et al. Cardiovascular Disease-Related Morbidity and Mortality in Women with a History of Pregnancy Complications. Circulation 2019, 139, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Lane-Cordova, A.D.; Khan, S.S.; Grobman, W.A.; Greenland, P.; Shah, S.J. Long-Term Cardiovascular Risks Associated with Adverse Pregnancy Outcomes: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2106–2116. [Google Scholar] [CrossRef] [PubMed]
- Ridder, A.; Giorgione, V.; Khalil, A.; Thilaganathan, B. Preeclampsia: The Relationship between Uterine Artery Blood Flow and Trophoblast Function. Int. J. Mol. Sci. 2019, 20, 3263. [Google Scholar] [CrossRef] [PubMed]
- Cain, M.A.; Salemi, J.L.; Tanner, J.P.; Kirby, R.S.; Salihu, H.M.; Louis, J.M. Pregnancy as a window to future health: Maternal placental syndromes and short-term cardiovascular outcomes. Am. J. Obstet. Gynecol. 2016, 215, 484.e1–484.e14. [Google Scholar] [CrossRef]
- Petersen, E.E.; Davis, N.L.; Goodman, D.; Cox, S.; Syverson, C.; Seed, K.; Shapiro-Mendoza, C.; Callaghan, W.M.; Barfield, W. Racial/Ethnic Disparities in Pregnancy-Related Deaths—United States, 2007–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 762–765. [Google Scholar] [CrossRef]
- Maternal Mortality in the United States—AAF. Available online: https://www.americanactionforum.org/insight/maternal-mortality-in-the-united-states/ (accessed on 13 November 2021).
- Newborns: Improving Survival and Well-Being. Available online: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality (accessed on 9 November 2021).
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.-B.; Kinney, M.; Lawn, J.; The Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million preterm births. Reprod. Health 2013, 10 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Haas, D.M.; Parker, C.B.; Marsh, D.; Grobman, W.A.; Ehrenthal, D.B.; Greenland, P.; Merz, C.N.B.; Pemberton, V.L.; Silver, R.M.; Barnes, S.; et al. Association of Adverse Pregnancy Outcomes with Hypertension 2 to 7 Years Postpartum. J. Am. Heart Assoc. 2019, 8, e013092. [Google Scholar] [CrossRef]
- Crump, C.; Groves, A.; Sundquist, J.; Sundquist, K. Association of Preterm Birth with Long-term Risk of Heart Failure Into Adulthood. JAMA Pediatr. 2021, 175, 689–697. [Google Scholar] [CrossRef]
- Hauspurg, A.; Ying, W.; Hubel, C.A.; Michos, E.D.; Ouyang, P. Adverse pregnancy outcomes and future maternal cardiovascular disease. J. Clin. Cardiol. 2018, 41, 239–246. [Google Scholar] [CrossRef]
- Barrett, P.; McCarthy, F.P.; Kublickiene, K.; Cormican, S.; Judge, C.; Evans, M.; Kublickas, M.; Perry, I.J.; Stenvinkel, P.; Khashan, A.S. Adverse Pregnancy Outcomes and Long-term Maternal Kidney Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e1920964. [Google Scholar] [CrossRef]
- Egan, A.M.; Dunne, F.P. Epidemiology of Gestational and Pregestational Diabetes Mellitus. Front. Diabetes 2020, 28, 1–10. [Google Scholar] [CrossRef]
- Deputy, N.P.; Kim, S.Y.; Conrey, E.J.; Bullard, K.M. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes among Women Who Had a Live Birth—United States, 2012–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 67, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Domanski, G.; Lange, A.E.; Ittermann, T.; Allenberg, H.; Spoo, R.A.; Zygmunt, M.; Heckmann, M. Evaluation of neonatal and maternal morbidity in mothers with gestational diabetes: A population-based study. BMC Pregnancy Childbirth 2018, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Mitanchez, D.; Weill, A.; Nizard, J.; Alla, F.; Hartemann, A.; Jacqueminet, S. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017, 60, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Kc, K.; Shakya, S.; Zhang, H. Gestational Diabetes Mellitus and Macrosomia: A Literature Review. Ann. Nutr. Metab. 2015, 66 (Suppl. S2), 14–20. [Google Scholar] [CrossRef]
- Gorban de Lapertosa, S.; Alvariñas, J.; Elgart, J.F.; Salzberg, S.; Gagliardino, J.J. The triad macrosomia, obesity, and hypertriglyceridemia in gestational diabetes. Diabetes Metab. Res. Rev. 2020, 36, e03302. [Google Scholar] [CrossRef]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Dunne, F.; Lawlor, D.A. Hyperglycaemia and risk of adverse perinatal outcomes: Systematic review and meta-analysis. Br. Med. J. 2016, 354, i4694. [Google Scholar] [CrossRef]
- Parikh, N.I.; Gonzalez, J.M.; Anderson, C.A.; Judd, S.E.; Rexrode, K.M.; Hlatky, M.A.; Gunderson, E.P.; Stuart, J.J.; Vaidya, D. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement from the American Heart Association. Circulation 2021, 143, E902–E916. [Google Scholar] [CrossRef]
- Dabelea, D.; Pettitt, D.J. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J. Pediatr. Endocrinol. Metabol. 2001, 14, 1085–1091. [Google Scholar] [CrossRef]
- Boston Birth Cohort Study—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03228875 (accessed on 10 November 2021).
- Wang, X.; Zuckerman, B.; Pearson, C.; Kaufman, G.; Chen, C.; Wang, G.; Bauchner, H.; Wise, P.H.; Niu, T.; Xu, X. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA 2002, 287, 195–202. [Google Scholar] [CrossRef]
- McCarty-Singleton, S.; Sciscione, A. Committee on Practice Bulletins-Obstetrics, The American College of, Obstetricians. Practice bulletin No. 130: Prediction and prevention of preterm birth. Obstet. Gynecol. 2012, 120, 964–973. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program. Working Group Report on High Blood Pressure in Pregnancy. Am. J. Obstet. Gynecol. 1990, 163 Pt 1, 1691–1712. [Google Scholar] [CrossRef]
- American Diabetes Association. Gestational Diabetes Mellitus. Diabetes Care 2003, 26 (Suppl. S1), s103–s105. [Google Scholar] [CrossRef] [PubMed]
- Committee on Practice Bulletins—Obstetrics ACOG. Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol. 2018, 131, e49–e64. [Google Scholar] [CrossRef] [PubMed]
- HAPO Study Cooperative Research Group; Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hod, M.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- The extended Pedersen Hypothesis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/3402161/ (accessed on 14 November 2021).
- Köck, K.; Köck, F.; Klein, K.; Bancher-Todesca, D.; Helmer, H. Diabetes mellitus and the risk of preterm birth with regard to the risk of spontaneous preterm birth. J. Matern. Fetal. Neonatal. Med. 2010, 23, 1004–1008. [Google Scholar] [CrossRef] [PubMed]
- Bryson, C.L.; Ioannou, G.N.; Rulyak, S.J.; Critchlow, C. Association between gestational diabetes and pregnancy-induced hypertension. Am. J. Epidemiol. 2003, 158, 1148–1153. [Google Scholar] [CrossRef]
- Seely, E.W.; Solomon, C.G. Insulin resistance and its potential role in pregnancy-induced hypertension. J. Clin. Endocrinol. Metabol. 2003, 88, 2393–2398. [Google Scholar] [CrossRef]
- Mastrogiannis, D.S.; Spiliopoulos, M.; Mulla, W.; Homko, C.J. Insulin resistance: The possible link between gestational diabetes mellitus and hypertensive disorders of pregnancy. Curr. Diab. Rep. 2009, 9, 296–302. [Google Scholar] [CrossRef]
- Negrato, C.A.; Jovanovic, L.; Tambascia, M.A.; Geloneze, B.; Dias, A.; Calderon, I.D.M.P.; Rudge, M.V.C. Association between insulin resistance, glucose intolerance, and hypertension in pregnancy. Metab. Syndr. Relat. Disord. 2009, 7, 53–59. [Google Scholar] [CrossRef]
- Hoodbhoy, Z.; Mohammed, N.; Nathani, K.R.; Sattar, S.; Chowdhury, D.; Maskatia, S.; Tierney, S.; Hasan, B.; Das, J.K. The impact of maternal preeclampsia and hyperglycemia on the cardiovascular Health of the offspring: A systematic Review and meta-analysis. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Ghosh, G.; Grewal, J.; Männistö, T.; Mendola, P.; Chen, Z.; Xie, Y.; Laughon, S.K. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn. Dis. 2014, 24, 283. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4171100/ (accessed on 15 November 2021).
- Tanaka, M.; Jaamaa, G.; Kaiser, M.; Hills, E.; Soim, A.; Zhu, M.; Shcherbatykh, I.Y.; Samelson, R.; Bell, E.; Zdeb, M.; et al. Racial disparity in hypertensive disorders of pregnancy in New York state: A 10-year longitudinal population-based study. Am. J. Public Health 2007, 97, 163–170. [Google Scholar] [CrossRef]
- Racial Disparities Persist in Maternal Morbidity, Mortality and Infant Health. Available online: https://www.ajmc.com/view/racial-disparities-persist-in-maternal-morbidity-mortality-and-infant-health (accessed on 15 November 2021).
- Okosun, I.S.; Chandra, K.; Boev, A.; Boltri, J.M.; Choi, S.T.; Parish, D.C.; Dever, G. Abdominal adiposity in U.S. adults: Prevalence and trends, 1960–2000. Prev. Med. 2004, 39, 197–206. [Google Scholar] [CrossRef]
- Lao, T.T.; Ho, L.-F.; Chan, B.C.; Leung, W.-C. Maternal Age and Prevalence of Gestational Diabetes Mellitus. Diabetes Care 2006, 29, 948–949. [Google Scholar] [CrossRef]
- Jeyabalan, A. Epidemiology of preeclampsia: Impact of obesity. Nutr. Rev. 2013, 71 (Suppl. S1), S18–S25. [Google Scholar] [CrossRef]
- Hadar, E.; Oats, J.; Hod, M. Towards new diagnostic criteria for diagnosing GDM: The HAPO study. J. Perinatal. Med. 2009, 37, 447–449. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Colagiuri, S.; Roglic, G.; Hod, M. Diagnosis of GDM: A suggested consensus. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 194–205. [Google Scholar] [CrossRef]


| Maternal and Birth Characteristics | Maternal Glycemic Subgroups during Pregnancy (Mutually Exclusive) | p-Value | ||||
|---|---|---|---|---|---|---|
| without PDM or GDM | GDM | PDM | ||||
| T1 of 1 h Glucose (50–95 mg/dL) n = 1166 | T2 of 1 h Glucose (96–116 mg/dL) n = 1151 | T3 of 1 h Glucose (117–201 mg/dL) n = 913 | n = 582 | n = 307 | ||
| Age, n (%) | <0.001 | |||||
| <20 | 158 (13.6) | 111 (9.6) | 66 (7.2) | 16 (2.8) | 14 (4.5) | |
| 20–34 | 875 (75.0) | 869 (75.5) | 639 (70.0) | 393 (67.5) | 201 (65.5) | |
| ≥35 | 133 (11.4) | 171 (14.9) | 208 (22.8) | 173 (29.7) | 92 (30.0) | |
| Race, n (%) | <0.001 | |||||
| Non-Hispanic Black | 499 (42.8) | 435 (37.8) | 307 (33.6) | 163 (28.0) | 111 (36.2) | |
| Hispanic | 156 (13.4) | 186 (16.2) | 142 (15.6) | 150 (25.8) | 66 (21.5) | |
| Others * | 511 (43.8) | 530 (46.1) | 464 (50.8) | 269 (46.2) | 130 (42.4) | |
| Parity, n (%) | <0.001 | |||||
| 0 | 595 (51.0) | 528 (45.9) | 373 (40.9) | 184 (31.6) | 110 (35.8) | |
| ≥1 | 571 (49.0) | 623 (54.1) | 540 (59.2) | 398 (68.4) | 197 (64.2) | |
| Educational Status, n (%) | 0.060 | |||||
| Secondary or less | 291 (25.0) | 257 (22.3) | 224 (24.5) | 174 (29.9) | 88 (28.7) | |
| GED/High school | 402 (34.5) | 409 (35.5) | 307 (33.6) | 183 (31.4) | 94 (30.6) | |
| College and above | 473 (40.5) | 485 (42.2) | 382 (41.9) | 225 (38.7) | 125 (40.7) | |
| Pre-existing Hypertension, n (%) | <0.001 | |||||
| No | 1133 (97.2) | 1091 (94.8) | 856 (93.7) | 526 (90.4) | 234 (76.2) | |
| Yes | 33 (2.8) | 60 (5.2) | 57 (6.3) | 56 (9.6) | 73 (23.8) | |
| Smoking in pregnancy, n (%) | 0.004 | |||||
| No | 994 (85.2) | 1023 (88.9) | 809 (88.6) | 527 (90.6) | 260 (84.7) | |
| Yes | 172 (14.8) | 128 (11.1) | 104 (11.4) | 55 (9.5) | 47 (15.3) | |
| Body Mass Index, n (%) | <0.001 | |||||
| <25 | 665 (57.0) | 556 (48.3) | 413 (45.2) | 159 (27.3) | 72 (23.5) | |
| 25–29.9 | 280 (24.0) | 324 (28.2) | 241 (26.4) | 172 (29.6) | 91 (29.6) | |
| ≥30 | 155 (13.3) | 208 (18.1) | 209 (22.9) | 212 (36.4) | 121 (39.4) | |
| Missing | 66 (5.7) | 63 (5.5) | 50 (5.5) | 39 (6.7) | 23 (7.5) | |
| Sex of infant, n (%) | 0.514 | |||||
| Female | 588 (50.4) | 565 (49.1) | 453 (49.6) | 294 (50.5) | 138 (45.0) | |
| Male | 578 (49.6) | 586 (50.9) | 460 (50.4) | 288 (49.5) | 169 (55.0) | |
| Gestational age(weeks), n (%) | <0.001 | |||||
| <37 | 235 (20.2) | 240 (20.8) | 189 (20.7) | 192 (33.0) | 132 (43.0) | |
| ≥37 | 931 (79.8) | 911 (79.2) | 724 (79.3) | 390 (67.0) | 175 (57.0) | |
| Birthweight (g), n (%) | <0.001 | |||||
| <4000 | 1120 (96.1) | 1080 (93.8) | 845 (92.6) | 500 (85.9) | 285 (92.8) | |
| ≥4000 | 46 (4.9) | 71 (6.2) | 68 (7.4) | 82 (14.1) | 22 (7.2) | |
| Maternal Glycemic Subgroups with/without Pre-Existing Hypertension | Crude | Adjusted * | ||
|---|---|---|---|---|
| OR [95%CI] | p-Value | OR [95%CI] | p-Value | |
| T1 with no pre-existing hypertension | reference | reference | ||
| T2 with no pre-existing hypertension | 1.50 [1.14–1.97] | 0.003 | 1.47 [1.11–1.93] | 0.007 |
| T3 with no pre-existing hypertension | 1.44 [1.09–1.90] | 0.011 | 1.37 [1.03–1.82] | 0.031 |
| T1 with pre-existing hypertension | 8.83 [4.51–17.29] | <0.001 | 7.63 [3.81–15.30] | <0.001 |
| T2 with pre-existing hypertension | 7.18 [4.16–12.39] | <0.001 | 5.91 [3.36–10.40] | <0.001 |
| T3 with pre-existing hypertension | 7.59 [4.62–12.46] | <0.001 | 6.59 [3.89–11.17] | <0.001 |
| Maternal Glycemic Subgroups | Crude | Adjusted * | ||
|---|---|---|---|---|
| OR [95%CI] | p-Value | OR [95%CI] | p-Value | |
| Non-Hispanic Black women (n = 1515) | ||||
| T1 | 1.00 [Ref] | 1.00 [Ref] | ||
| T2 | 1.82 [1.22–2.72] | 0.003 | 1.67 [1.13–2.51] | 0.013 |
| T3 | 1.87 [1.22–2.88] | 0.004 | 1.68 [1.07–2.62] | 0.023 |
| Gestational DM | 2.60 [1.60–4.21] | <0.001 | 2.09 [1.25–3.49] | <0.001 |
| Pregestational DM | 2.30 [1.31–4.04] | <0.001 | 1.84 [1.02–3.32] | <0.001 |
| Hispanic women (n = 700) | ||||
| T1 | 1.00 [Ref] | 1.00 [Ref] | ||
| T2 | 0.53 [0.25–1.13] | 0.102 | 0.54 [0.25–1.18] | 0.120 |
| T3 | 0.64 [0.29–1.41] | 0.273 | 0.58 [0.26–1.29] | 0.181 |
| Gestational DM | 1.84 [0.97–3.47] | 0.061 | 1.70 [0.82–3.52] | 0.151 |
| Pregestational DM | 2.66 [1.27–5.57] | 0.009 | 2.40 [1.07–5.39] | <0.001 |
| Women of Other Race-ethnicities† (n = 1904) | ||||
| T1 | 1.00 [Ref] | 1.00 [Ref] | ||
| T2 | 1.74 [1.17–2.58] | 0.006 | 1.63 [1.09–2.44] | 0.017 |
| T3 | 1.26 [0.82–1.93] | 0.302 | 1.14 [0.73–1.77] | 0.559 |
| Gestational DM | 3.19 [2.09–4.87] | <0.001 | 2.60 [1.68–4.05] | <0.001 |
| Pregestational DM | 3.13 [1.86–5.24] | <0.001 | 2.51 [1.47–4.30] | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwapong, Y.A.; Boakye, E.; Wang, G.; Hong, X.; Lewey, J.; Mamas, M.A.; Wu, P.; Blaha, M.J.; Nasir, K.; Hays, A.G.; et al. Maternal Glycemic Spectrum and Adverse Pregnancy and Perinatal Outcomes in a Multiracial US Cohort. J. Cardiovasc. Dev. Dis. 2022, 9, 179. https://doi.org/10.3390/jcdd9060179
Kwapong YA, Boakye E, Wang G, Hong X, Lewey J, Mamas MA, Wu P, Blaha MJ, Nasir K, Hays AG, et al. Maternal Glycemic Spectrum and Adverse Pregnancy and Perinatal Outcomes in a Multiracial US Cohort. Journal of Cardiovascular Development and Disease. 2022; 9(6):179. https://doi.org/10.3390/jcdd9060179
Chicago/Turabian StyleKwapong, Yaa Adoma, Ellen Boakye, Guoying Wang, Xiumei Hong, Jennifer Lewey, Mamas Andreas Mamas, Pensee Wu, Michael Joseph Blaha, Khurram Nasir, Allison Gamboa Hays, and et al. 2022. "Maternal Glycemic Spectrum and Adverse Pregnancy and Perinatal Outcomes in a Multiracial US Cohort" Journal of Cardiovascular Development and Disease 9, no. 6: 179. https://doi.org/10.3390/jcdd9060179
APA StyleKwapong, Y. A., Boakye, E., Wang, G., Hong, X., Lewey, J., Mamas, M. A., Wu, P., Blaha, M. J., Nasir, K., Hays, A. G., Blumenthal, R. S., Wang, X., & Sharma, G. (2022). Maternal Glycemic Spectrum and Adverse Pregnancy and Perinatal Outcomes in a Multiracial US Cohort. Journal of Cardiovascular Development and Disease, 9(6), 179. https://doi.org/10.3390/jcdd9060179