White Rot Fungi as Tools for the Bioremediation of Xenobiotics: A Review
Abstract
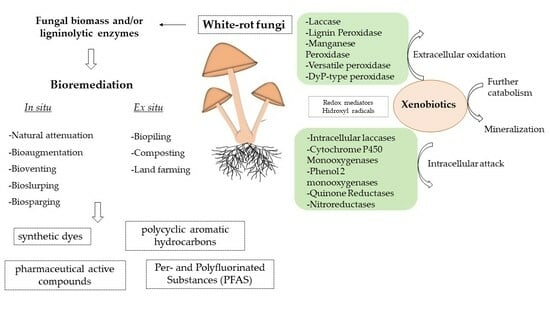
:1. Introduction
2. White Rot Fungi and the Degradation of Lignin
3. Main Enzymes Associated with the Degradation of Xenobiotics
3.1. Structure, Function and Applications of Laccase
3.2. Structure and Function of Fungal Class II Peroxidases
3.3. Lignin Peroxidases
3.4. Manganese Peroxidases
3.5. Versatile Peroxidases
3.6. Dye-Decolorizing Peroxidases
3.7. Fungal Monooxygenases: The Cytochrome P450 Monooxygenases
3.8. Biotechnological Applications of Ligninolytic Enzymes
| Enzyme | Localization | Reaction Mechanism, Substrates | Applications | Reference |
|---|---|---|---|---|
| Laccases (EC 1.10.3.2) benzene diol: oxygen oxidoreductase | Mainly extracellular Some intracellular | O2-dependent
|
| Baldrian [16] Giardina et al. [58] Sirim et al. [62] |
| Tyrosinases Grouped into two enzyme subclasses Oxidase (EC 1.10.3.1) and monooxygenase (EC 1.14.18.1) | Mainly intracellular or Cell-wall-associated |
|
| Hofrichter and Ulrich [103] |
| Lignin peroxidases (EC.1.11.1.14) diarylpropane: oxygen, H2O2, oxidoreductase | Extracellular |
|
| Husain et al. [160] Biko et al. [52] Chowdhary et al. [104] Singh et al. [14] |
| Manganese peroxidases (EC 1.11.1.13; Mn(II): H2O2 oxidoreductase) | Extracellular |
|
| Hofrichter [105]; Husain et al. [161] Kumar and Arora [156] Bilal et al. [159] |
| Versatile peroxidases (EC 1.11.1.16) Reactive Black 5:H2O2 oxidoreductase) | Extracellular |
|
| Pérez-Boada et al. [112] Ruiz-Dueñas et al. [101] Barber-Zucker et al. [162] |
| DyP-type peroxidases (EC 1.11.1.19) | Extracellular |
|
| Puhse et al. [130] Colpa et al. [124] Xu et al. [163] |
| Cytochrome P450 Monooxygenases (EC 1.14.14.1) | Cell bound |
|
| Young et al. [141] Mori et al. [143] |
| Phenol 2 monooxygenases (EC 1.14.13.7) | Cell bound |
|
| Hofricher and Ulrich [103] Harms et al. [155] |
| Nitroreductases (EC: 1.5.1.34) | Cell bound |
|
| Harms et al. [155] |
| Quinone Reductases (EC 1.6.99.2) | Cell bound |
|
| Harms et al. [155] |
4. Mechanisms Used by Fungi for the Degradation of Xenobiotics
5. Bioremediation by White Rot Fungi
5.1. Biodegradation of Synthetic Dyes and Textile Wastewater
5.2. Biodegradation of Polycyclic Aromatic Hydrocarbons
5.3. Biodegradation of Pharmaceutically Active Compounds (PhACs)
5.4. Biodegradation of Per- and Polyfluoroalkyl Substances
| White Rot Fungi | Pollutants & Conditions | Removal Rates | References |
|---|---|---|---|
| Synthetic dyes and textile wastewater | |||
| Pleurotus ostreatus |
|
| Pezzella et al. [82] |
|
| Dai et al. [189] | |
|
| Skariyachan et al. [190] | |
|
| Zhuo et al. [77] | |
|
| George et al. [191] | |
| Phanerochaete chrysosporium |
|
| Freire Andrade et al. [192] |
|
| Rani et al. [193] | |
|
| Li et al. [194] | |
|
| Wanderley et al. [195] | |
|
| Sierra-Solanche et al. [196] | |
|
| Oliveira Santos et al. [197] | |
| Decolorization percentages:
| Pereira de Almeida et al. [198] | |
| Ganoderma lucidum |
|
| Selvakumar et al. [203] |
|
| Ma et al. [204] | |
|
| Palazzolo et al. [202] | |
|
| Rainer et al. [205] | |
|
| Himanshu et al. [206] | |
| Ganoderma weberianum B-18 |
|
| Torres-Farradá et al. [207] |
| Polycyclic Aromatic Hydrocarbons | |||
| Phanerochaete chrysosporium |
| Degradation of phenanthrene: 99.55% under carbon-rich and 92.77% under nitrogen-limiting conditions Degradation of pyrene: 99.47% under carbon-rich and 83.97% under nitrogen-limiting conditions | Ding et al. [210] |
| Evaluation of the degradation of pyrene in different soils |
| Wang et al. [211] | |
| Pleurotus ostreatus |
|
| Pozdnyakova et al. [6] |
|
| Elhusseiny et al. [212] | |
| Pharmaceutically Active Compounds | |||
| Trametes versicolor |
|
| Marco-Urrea et al. [145] |
|
| Cruz-Morato et al. [220] | |
| Bjerkandera spp. TBB-03 |
|
| Bilal et al. [221] |
| Fomes fomentarius Hypholoma fasciculare T. versicolor |
|
| Jureczko et al. [218] |
| Per- and polyfluoroalkyl substances | |||
| Pleurotus ostreatus |
|
| Luo et al. [240] |
| Phanerochaete chrysosporium Aspergillus niger Five fungal strains isolated from contaminated site with PFASs |
|
| Tseng et al. [241] |
| Gloephyllum trabeum Trametes versicolor Six fungal isolates from a location contaminated with PFASs |
|
| Merino et al. [242] |
6. Current Limitations of Using WRF for the Bioremediation of Polluted Environments and Future Strategies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asgher, M.; Bhatti, H.; Ashraf, M.; Legge, R. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Subbulakshmi, G.; Debbarma, A.; Sinha, A.; Panda, S. Bio remediation of xenobiotic compound: Reclamation approach for environmental sustainability—A review. Mater.Today Proc. 2021, 47, 1108–1113. [Google Scholar] [CrossRef]
- Ramaka, S.; Vasudeva, M.S.; Rao, A.R. Xenobiotics in health and disease: The two sides of a coin: A clinician perspective. J. Toxicol. 2020, 4, 555–641. [Google Scholar]
- Kathhivaran, A.; Gnanadoss, J.J. White rot fungi-mediated bioremediation as a sustainable method for xenobiotic degradation. Environ. Exp. Biol. 2021, 19, 103–119. [Google Scholar]
- Chen, S.; Zhu, M.; Guo, X.; Yang, B.; Zhuo, R. Coupling of fenton reaction and white rot fungi for the degradation of organic pollutants. Ecotoxicol. Environ. Saf. 2023, 254, 114697. [Google Scholar] [CrossRef]
- Pozdnyakova, N.N.; Chernyshovaa, M.P.; Grineva, V.S.; Landesmanb, E.O.; Turkovskayaa, V. Degradation of Fluorene and Fluoranthene by the Basidiomycete Pleurotus ostreatus. Appl. Biochem. Microbiol. 2016, 52, 621–628. [Google Scholar] [CrossRef]
- Vanhulle, S.; Trovaslet, M.; Enaud, E.; Lucas, M.; Taghavi, S.; Van der Lelie, D.; van Aken, B.; Foret, M.; Onderwater, R.C.A.; Wesenberg, D.; et al. Decolorization, cytotoxicity, and genotoxicity reduction during a combined ozonation/fungal treatment of dye contaminated wastewater. Environ. Sci. Technol. 2008, 42, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Ijoma, G.N.; Tekere, M. Potential microbial applications of co-cultures involving ligninolytic fungi in the bioremediation of recalcitrant xenobiotic compounds. Int. J. Environ. Sci. Technol. 2017, 14, 1787–1806. [Google Scholar] [CrossRef]
- Stefanac, T.; Gragas, D.; Dragicevic, T.L. Xenobiotics–division and methods of detection: A review. J. Xenobiot. 2021, 11, 130–141. [Google Scholar] [CrossRef]
- Nogacka, A.M.; Gómez-Martín, M.; Suárez, A.; González-Bernardo, O.; de los Reyes-Gavilán, C.; González, S. Xenobiotics formed during food processing: Their relation with the intestinal microbiota and colorectal cancer. Int. J. Mol. Sci. 2019, 20, 2051. [Google Scholar] [CrossRef]
- Aksu, Z. Application of biosorption for the removal of organic pollutants: A review. Process Biochem. 2005, 40, 997–1026. [Google Scholar] [CrossRef]
- Erkurt, E.A.; Unyayar, A.; Kumbur, H. Decolorization of synthetic dyes by white rot fungi, involving laccase enzyme in the process. Process Biochem. 2007, 42, 1429–1435. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of heavy-metal pollutants by white rot fungi: Mechanisms, achievements, and perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Singh, R.K.; Tripathi, R.; Ranjan, A.; Srivastava, A.K. Fungi as potential candidates for Bioremediation. In Abatement of Environmental Pollutants. Trends and Strategies, 1st ed.; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Winquist, E.; Björklöf, K.; Schultz, E.; Räsänen, M.; Salonen, K.; Anasonye, F.; Cajthaml, T.; Steffen, K.T.; Jørgensen, K.S.; Tuomela, M. Bioremediation of PAH-contaminated soil with fungi, from laboratory to field scale. Int. Biodeterior. Biodegr. 2014, 86, 238–247. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Latif, W.; Ciniglia, C.; Iovinella, M.; Shafiq, M.; Papa, S. Role of White Rot Fungi in Industrial Wastewater Treatment: A Review. Appl. Sci. 2023, 13, 8318. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A comprehensive insight into the application of white rot fungi and their lignocellulolytic enzymes in the removal of organic pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Stella, T.; Stefano, C.; Čvančarová, M.; Filipová, A.; Petruccioli, M.; D’Annibale, A.; Cajthaml, T. Bioremediation of long-term PCB-contaminated soil by white-rot fungi. J. Hazard. Mater. 2017, 324, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Harry-Asobara, J.L.; Kamei, I. Characteristics of white rot fungus Phlebia brevispora TMIC33929 and its growth-promoting bacterium enterobacter sp. TN3W-14 in the decolorization of dye-contaminated water. Appl. Biochem. Biotechnol. 2019, 189, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Yesilada, O.; Birhanli, E.; Geckil, H. Bioremediation and decolorization of textile dyes by white rot fungi and laccase. Enzymes. In Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Fungal Biology; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.-W.; Dong, C.-D. Organic wastes bioremediation and its changing prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2017, 51, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Premnath, N.; Mohanrasu, K.; Guru Raj Rao, R.; Dinesh, G.H.; Siva Prakash, G.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons—Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Imam, A.; Suman, S.K.; Vempatapu, B.P. Pyrene remediation by Trametes maxima: An insight into secretome response and degradation pathaway. Environ. Sci. Pollut. Res. 2022, 29, 44135–44147. [Google Scholar] [CrossRef]
- Akerman-Sanchez, G.; Rojas-Jimenez, K. Fungi for the bioremediation of pharmaceutical-derived pollutants: A bioengineering approach to water treatment. Environ. Adv. 2021, 4, 100071. [Google Scholar] [CrossRef]
- Amobonye, A.; Aruwa, C.E.; Aransiola, S.; Omame, J.; Alabi, T.D.; Lalung, J. the potential of fungi in the bioremediation of pharmaceutical active compounds: A comprehensive review. Front. Microbiol. 2023, 14, 1207792. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M.N. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants—A review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef]
- Sugano, Y.; Muramatsu, R.; Ichiyanagi, A.; Sato, T.; Shoda, M. DyP, a Unique Dye-decolorizing Peroxidase, Represents a Novel Heme Peroxidase Family Asp171 replaces the distal histidine of classical peroxidases. J. Biol. Chem. 2007, 282, 36652–36658. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Vilar, D.; Bilal, M.; Bharagava, R.N.; Kumar, A.; Nadda, A.K.; Salazar-Banda, G.R.; Barrios-Eguiluz, K.; Romanholo-Ferreira, L.F. Lignin-modifying enzymes: A Green and environmental responsive technology for organic compound degradation. J. Chem. Technol. Biotechnol. 2021, 97, 327–342. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Baccar, R.; Caminal, G.; Sarrà, M. Can white-rot fungi be a real wastewater treatment alternative for organic micropollutants removal? A review. Water Res. 2018, 138, 137–151. [Google Scholar] [CrossRef]
- Béla Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Yong, H.N.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef]
- Dávila, G.; Vázquez-Duhalt, R. Enzimas ligninolíticas fúngicas para fines ambientales. Mensaje Bioquímico 2006, 41, 29–55. [Google Scholar]
- Kirk, P.; Cannon, P.; Minter, D.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008; ISBN 4780851998268. [Google Scholar]
- Hybelbauerová, S.; Sejbal, J.; Dracínský, M.; Hahnová, A.; Koutek, B. Chemical constituents of Stereum subtomentosum and two other birch-associated basidiomycetes: An interspecies comparative study. Chem. Biodivers. 2008, 5, 743–750. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L.; Martinez, A.I.; León-Ramírez, C.; Sentandreu, R. Analysis of the proteins involved in the structure and synthesis of the cell wall of Ustilago maydis. Fungal Genet. Biol. 2008, 45, 71–76. [Google Scholar] [CrossRef]
- Webster, J.; Weber, R. Introduction to Fungi, 3rd ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. 2020, 95, 481–494. [Google Scholar] [CrossRef]
- Wymelenberg, A.V.; Gaskell, J.; Mozuch, M.; Sabat, G.; Ralph, J.; Skyba, O.; Mansfield, S.D.; Blanchette, R.A.; Martinez, D.; Grigoriev, I.; et al. Comparative Transcriptome and Secretome Analysis of Wood Decay Fungi Postia placenta and Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2010, 76, 3599–3610. [Google Scholar] [CrossRef]
- Fukasawa, Y.; Kaga, K. Surface Area of Wood Influences the Effects of Fungal Interspecific Interaction on Wood Decomposition—A Case Study Based on Pinus densiflora and Selected White Rot Fungi. J. Fungi 2022, 8, 517. [Google Scholar] [CrossRef]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R. The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes. Science 2012, 336, 9. [Google Scholar] [CrossRef]
- Riley, R.; Salamov, A.A.; Brown, D.W.; Grigoriey, I.V. Extensive sampling of basidiomycete genomes demonstrates inadequacy of the white-rot/brown-rot paradigm for wood decay fungi. Proc. Natl. Acad. Sci. USA 2014, 11, 9923–9928. [Google Scholar] [CrossRef]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Dohra, H.; Suzuki, T.; Kawagishi, H.; Hirai, H. Draft genome sequence of the white-rot fungus Phanerochaete sordida YK-624. Microbiol. Resour. Announc. 2021, 10, 00842-21. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Wang, J.; Wang, Z.; Zhan, H.; Yu, X.; Zheng, Y.; Xiao, T.; Zhou, L.-W. Biodegradation of Benzo[a]pyrene by a White-Rot Fungus Phlebia acerina: Surfactant- Enhanced Degradation and Possible Genes Involved. J. Fungi 2023, 9, 978. [Google Scholar] [CrossRef]
- Hou, L.; Ji, D.; Dong, W.; Yuan, L.; Zhang, F.; Li, Y.; Zang, L. The Synergistic Action of Electro-Fenton and White-Rot Fungi in the Degradation of Lignin. Front. Bioeng. Biotechnol. 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, M.; Oguiza, J.A.; Ramírez, L.; Pisabarro, A.G. Comparative analysis of secretomes in basidiomycete fungi. J. Proteom. 2014, 102, 28–43. [Google Scholar] [CrossRef]
- Wesenberg, D.; Kyriakides, I.; Agathos, S. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 2023, 22, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Biko, O.D.V.; Viljoen-Bloom, M.; van Zyl, W.H. Microbial lignin peroxidases: Applications, production challenges and future perspectives. Enzyme Microb. Technol. 2020, 141, 109669. [Google Scholar] [CrossRef] [PubMed]
- Songulashvili, G.; Elisashvili, V.; Wasser, S.P.; Nevo, E.; Hadar, Y. Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry wastes. Enzyme Microb. Technol. 2007, 41, 57–61. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Karhunen, E.; Salola, P.; Raunio, V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem. J. 1988, 254, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wasilewska, M.; Cho, N.; Hofrichter, M.; Rogalski, J. Biodegradation of lignin white-rot fungi: Review. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef]
- Janusz, G.; Kucharzykb, K.; Pawlika, A.; Staszczaka, M.; Paszczynskic, A. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzyme Microb. Technol. 2013, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.S.; Hedger, J.N. Degradation of plant cell Wall polymers. In Fungi in Bioremediation; Gadd, G.M., Ed.; British Mycological Society: Manchester, UK; Cambridge University Press: Cambridge, UK, 2001; pp. 1–20. [Google Scholar]
- Giardina, P.; Faraco, V.; Pezzella, C.; Pisutelli, A.; Vanhulle, S.; Sannnia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Latif, F.; Aziz, S.; Iqbal, R.; Iram, S.; Nazir, M.; Shakeel, M. Impact of pesticide application on aquatic environments and biodiversity. In Xenobiotics in Aquatic Animal Rather; Amin, M.A., Hajam, Y.A., Jamwal, A., Ahamd, I., Eds.; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Yoshida, H. LXIII.—Chemistry of lacquer (Urushi). Part I. Communication from the chemical society of Tokio. J. Chem. Soc. Trans. 1883, 43, 472–486. [Google Scholar] [CrossRef]
- Bertrand, G. Sur la presence simultanée de la laccase et de la tyrosinase dans le sue de quelques champignons. CR Hebd Seances Acad. Sci. 1896, 123, 463–465. [Google Scholar]
- Sirim, D.; Wagner, F.; Wang, L.; Schmid, R.D.; Pleiss, J. The laccase engineering database: A classification and analysis system for laccases and related multicopper oxidases. Database 2011, 2011, bar006. [Google Scholar] [CrossRef]
- Singh, D.; Gupta, N. Microbial Laccase: A robust enzyme and its industrial applications. Biologia 2020, 75, 1183–1193. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Swiderska-Burek, U.; Polak, L.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent developments of a co-immobilized laccase–mediator system: A review. RSC Adv. 2021, 11, 29498. [Google Scholar] [CrossRef]
- Shleev, S.V.; Morozova, O.; Nikitina, O.; Gorshina, E.S.; Rusinova, T.; Serezhenkov, V.A.; Burbaev, D.; Gazaryan, I.; Yaropolov, A. Comparison of physico-chemical characteristics of four laccases from different basidiomycetes. Biochimie 2004, 86, 693–703. [Google Scholar] [CrossRef]
- Awasthi, M.; Jaiswal, N.; Singh, S.; Pandey, V.P.; Dwivedi, U.N. Molecular docking and dynamics simulation analyses unraveling the differential enzymatic catalysis by plant and fungal laccases with respect to lignin biosynthesis and degradation. J. Biomol. Struct. Dyn. 2015, 33, 1835–1849. [Google Scholar] [CrossRef] [PubMed]
- Tochhawng, L.; Kumar Mishra, V.; Pratap Singh, B. Endophytic fungi: Role in dye decolorization. In Advances in Endophytic Fungal Research; Singh, B., Ed.; Fungal Biology; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Ben-Younes, S.; Mechichi, T.; Sayadi, S. Purification and characterization of the laccase secreted by the white rot fungus Perenniporia tephropora and its role in the decolourization of synthetic dyes. J. Appl. Microbiol. 2007, 102, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Parra-Guardado, A.L.; Belleville, M.P.; Rostro-Alanis, M.J.; Parra-Saldivar, R.; Sanchez-Marcano, J. Effect of redox mediators in pharmaceuticals degradation by laccase: A comparative study. Process Biochem. 2019, 78, 123–131. [Google Scholar] [CrossRef]
- Saha, R.; Mukhopadhyay, M. Electrochemical analysis of Catechol polymerization in presence of Trametes versicolor laccase and the mediator ABTS. Enzyme Microb. Technol. 2021, 152, 109934. [Google Scholar] [CrossRef] [PubMed]
- Mateljak, I.; Monza, E.; Lucas, M.F.; Guallar, V.; Aleksejeva, O.; Ludwing, R.; Leech, D.; Shleev, S.; Alcalde, M. Increasing redox potential, redox mediator activity and stability in a fungal laccase by computer-guided mutagenesis and directed evolution. ACS Catal. 2019, 9, 4561–4572. [Google Scholar] [CrossRef]
- Fu, K.; Fan, L.; Luo, X. Identification of laccase genes in Trichoderma asperellum Ts93. Biotechnol. Lett. 2023, 45, 479–487. [Google Scholar] [CrossRef]
- Jafari, M.; Mojtabavi, S.; Faramarzi, M.A.; Mehernejad, F.; Soleimani, M.; Mirjani, R. Molecular level insight into stability, activity and structure of laccase in aqueous ionic liquid and organic solvents: An experimental and computational research. J. Mol. Liq. 2020, 317, 113925. [Google Scholar] [CrossRef]
- Strong, P.J.; Claus, H. Laccase: A review of its past and its future in bioremediation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 373–434. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-A resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Zhuo, R.; Zhang, J.; Yu, H.; Ma, F.; Zhang, X. The roles of Pleurotus ostreatus HAUCC 162 laccase isoenzymes in decolorization of synthetic dyes and the transformation pathways. Chemosphere 2019, 234, 733–745. [Google Scholar] [CrossRef]
- Giardina, P.; Cannio, R.; Martirani, L.; Marzullo, L.; Palmieri, G.; Sannia, G. Cloning and sequencing of a laccase gene from the lignin degrading Basidiomycete Pleurotus ostreatus. Appl. Environ. Microbiol. 1995, 61, 2408–2413. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Giardina, P.; Bianco, C.; Scaloni, A.; Capasso, A.; Sannia, G. A novel white laccase from Pleurotus ostreatus. J. Biol. Chem. 1997, 272, 31301–31307. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Palmieri, G.; Scaloni, A.; Fontanella, B.; Faraco, V.; Cennamo, G.; Sannia, G. Protein and gene structure of a blue laccase from Pleurotus ostreatus. Biochem. J. 1999, 341, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, G.; Cennamo, G.; Faraco, V.; Amoresano, A.; Sannia, G.; Giardina, P. Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme Microbiol. Technol. 2003, 33, 220–230. [Google Scholar] [CrossRef]
- Pezella, C.; Lettera, V.; Piscitelli, A.; Giardina, P.; Sannia, G. Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl. Microbiol. Biotechnol. 2013, 97, 705–717. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Pentari, C.; Termentzi, A.; America, A.H.P.; Zouraris, D.; Bhattachrya, S.K.; Karantonis, A.; Zervakis, G.I.; Topakas, E. Discovery of two novel laccase-like multicopper oxidases from Pleurotus citrinopileatus and their application in phenolic oligomer synthesis. Biotechnol. Biofuels 2021, 14, 83. [Google Scholar] [CrossRef]
- Wood, D.A. Production, purification and properties of extracellular laccase of Agaricus bisporus. J. Gen. Microbiol. 1980, 117, 327–338. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Raaska, L.; Itavaara, M. Detection of White rot fungi by a non-toxic stain. Mycol. Res. 1990, 94, 27–31. [Google Scholar] [CrossRef]
- Gindilis, V.; Goltsman, E.; Verlinsky, Y. Evolutionary classification of homeodomains. J. Assist. Reprod. Genet. 1998, 15, 349–377. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, B.X.; Wang, F.L.; Xin, L.; Gao, Y.; Ding, W.; He, X.M.; Liu, D.; Hu, X.M. Efficient lignin degradation of corn stalk by Trametes with high laccase activity and enzymatic stability in salt and ionic liquid. BioResources 2019, 14, 5339–5354. [Google Scholar] [CrossRef]
- Eggert, C.; Temp, U.; Eriksson, K.E. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: Purification and characterization of the laccase. Appl. Environ. Microbiol. 1996, 62, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.K.; Vekata Mohan, S.; Rao, R.; Ranjan Pati, B.; Sarma, P.N. Laccase production by Pleurotus ostreatus 1804: Optimization of submerged culture conditions by Taguchi DOE methodology. Biochem. Eng. J. 2005, 24, 17–26. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G.; Reid, I.D.; Lanthier, P.; Yaguchi, M. Lignin oxidation by Laccase isozymes from Trametes versicolor and role of mediator 2,2′azinobis (3-ethykbenzthiaozoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 1995, 61, 1876–1880. [Google Scholar] [CrossRef]
- Sánchez-López, M.I.; Guerra, G.; Hechevarria, Y.; Domínguez, O.; Manzano, A.M.; Torres, G.; Arguelles, J.; Ramos-Leal, M. Estabilidad y actividad enzimática del crudo enzimático de cultivo de Trametes maxima, decoloración in vitro de colorantes sintéticos. CNIC 2010, 41, 1–13. [Google Scholar]
- Ko, E.-M.; Leem, Y.-E.; Choi, H.T. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2001, 57, 98–102. [Google Scholar] [PubMed]
- Kumar, A.; Kant, K.; Kumar, P.; Ramchiary, N. Laccase isozymes from Ganoderma lucidum MDU-7: Isolation, characterization, catalytic properties and differential role during oxidative stress. J. Mol. Catal. Enzymat. 2015, 113, 68–75. [Google Scholar] [CrossRef]
- Teerapatsakul, C.; Abe, N.; Bucke, C.; Chitradon, L. Novel laccases of Ganoderma sp. KU-Alk4, regulated by different glucose concentration in alkaline media. World J. Microbiol. Biotechnol. 2007, 23, 1559–1567. [Google Scholar] [CrossRef]
- Torres-Farradá, G.; Manzano León, A.M.; Rineau, F.; Ledo Alonso, L.; Sánchez-López, M.I.; Thijs, S.; Colpaert, J.; Ramos- Leal, M.; Guerra, G.; Vangronsveld, J. Diversity of ligninolytic enzymes and their genes in strains of the genus Ganoderma: Applicable for biodegradation of xenobiotoc compounds? Front. Microbiol. 2017, 8, 898. [Google Scholar] [CrossRef]
- D’Souza-Ticlo, D.; Dharma, D.; Raghukumar, C.A. Thermostable metal-tolerant laccase with bioremediation potential from a martine-derived fungus. Mar. Biotechnol. 2009, 11, 725–737. [Google Scholar] [CrossRef]
- Divya, K.S.; Chouhan, J.B. Study of fungal diversity with reference to physical and chemical parameters. Int. J. Environ. Sci. 2014, 5, 401–406. [Google Scholar]
- Wikee, S.; Hatton, J.; Turbé-Doan, A.; Mathiue, Y.; Daou, M.; Lomascolo, A.; Kumar, A.; Lumyong, S.; Sciara, G.; Faulds, C.B.; et al. Characterization and dye decolorization potential of two laccases from the marine-derived fungus Pestalotioposis sp. Int. J. Mol. Sci. 2019, 20, 1864. [Google Scholar] [CrossRef]
- Welinder, K. Plant peroxidases: Structure–function relationships. In Plant Peroxidases, Topics and Detailed Literature on Molecular, Biochemical and Physiological Aspects; Penel, C., Gaspar, T., Greppin, H., Eds.; Université de Genève: Genève, Switzerland, 1992; pp. 1–24. [Google Scholar]
- Martínez, A.T.; Ruiz-Dueñas, F.J.; Martínez, M.J.; Del Rio, J.C.; Gutierrez, A. Enzymatic delignification of plant cell wall: From nature to mill. Curr. Opin. Biotechnol. 2009, 20, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Dueñas, F.; Morales, M.; García, E.; Miki, Y.; Martinez, M.; Martínez, A. Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases. J. Exp. Bot. 2009, 60, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bueno, N.S.; Suárez-Rodríguez, R.; Balcázar-López, E.; Folch-Mallo, J.L.; Ramírez-Trujillo, J.A.; Iturriaga, G. A versatile peroxidase from the fungus Bjerkandera adusta confers abiotic stress tolerance in transgenic tobacco plants. Plants 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M.; Ullrich, R.; Pecyna, M.; Liers, C.; Lundell, T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010, 87, 871–897. [Google Scholar] [CrossRef]
- Chowdhary, P.; Shukla, G.; Raj, G.; Romamholo Ferreira, L.F.; Naresh Bharagava, R. Microbial manganese peroxidase: A ligninolytic enzyme and its ample opportunities in research. SN Appl. Sci. 2019, 1, 45. [Google Scholar] [CrossRef]
- Hofrichter, M. Review: Lignin conversion by manganese peroxidase (MnP). Enzyme Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Gerini, M.F.; Roccatano, D.; Baciocchi, E.; Nola, A. Molecular Dynamics Simulations of Lignin Peroxidase in Solution. Biophys. J. 2003, 84, 3883–3893. [Google Scholar] [CrossRef]
- Civzele, A.; Stipniece-Jekimova, A.A.; Mezule, L. Fungal Ligninolytic Enzymes and Their Application in Biomass Lignin Pretreatment. J. Fungi 2023, 9, 780. [Google Scholar] [CrossRef]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Salman Ashraf, S. Laccases and peroxidases: The smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Martínez, A.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillen, F.; Martínez, M.J.; Gutiérrez, A.; Del Río, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar]
- Poulos, T.L.; Edwards, S.L.; Wariishi, H.; Gold, M.H. Crystallographic refinement of lignin peroxidase at 2 A. J. Biol. Chem. 1993, 268, 4429–4440. [Google Scholar] [CrossRef]
- Sánchez-Ruiz, M.I.; Ayuso-Fernández, I.; Rencoret, J.; González-Ramírez, A.M.; Linde, D.; Davó-Siguero, I.; Romero, A.; Gutierrez, A.; Martínez, A.T.; Ruiz-Dueñas, F.J. Agaricales mushroom liginin peroxidase: From structure to degradative capabilities. Antioxidants 2021, 10, 1446. [Google Scholar] [CrossRef]
- Pérez-Boada, M.; Ruiz-Dueñas, F.; Pogni, R.; Basosi, R.; Choinowski, T.; María Jesús Martínez, M. Versatile peroxidase oxidation of high redox potential aromatic compounds: Site-directed mutagenesis, spectroscopic and crystallographic investigation of three long-range electron transfer pathways. J. Mol. Biol. 2005, 354, 385–402. [Google Scholar] [CrossRef]
- Morgenstern, I.; Robertson, D.L.; Hibbett, D.S. Characterization of Three mnp Genes of Fomitiporia mediterranea and Report of Additional Class II Peroxidases in the Order Hymenochaetales. Appl. Environ. Microbiol. 2010, 76, 6431–6440. [Google Scholar] [CrossRef]
- Sáez-Jiménez, V.; Rencoret, J.; Rodríguez-Carvajal, M.A.; Gutiérrez, A.; Ruiz-Dueñas, F.J.; Martínez, A.T. Role of surface tryptophan for peroxidase oxidation of nonphenolic lignin. Biotechnol. Biofuels 2016, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q. Potential Applications of the oxidoreductive enzymes in the decolorization and detoxification of textile and other synthetic dyes from polluted water: A review. Crit. Rev. Biotechnol. 2006, 26, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, A.; Hammel, K.E. Fungal biodegradation of lignocelluloses. In The Mycota, X, Industrial Applications, 2nd ed.; Hofrichter, M., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2010; in press. [Google Scholar]
- Martínez, A.T. Molecular biology and structure-function of lignin degrading heme peroxidases. Enzyme Microb. Technol. 2002, 30, 425–444. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of four major mycotixins by eight manganese peroxidases in presence of a dicarboxylic acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef]
- Sundaramoorthy, M.; Youngs, H.L.; Gold, M.H.; Poulos, T.L. High resolution crystal structure of manganese peroxidase: Substrate and inhibitor complexes. Biochemistry 2005, 44, 6463–6470. [Google Scholar] [CrossRef]
- Gold, M.H.; Youngs, H.L.; Gelpke, M.D. Manganese peroxidase. Met. Ions Biol. Syst. 2000, 37, 559–586. [Google Scholar]
- Martínez, M.J.; Ruiz-Dueñas, F.J.; Martínez, A.T. Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur. J. Biochem. 1996, 237, 424–432. [Google Scholar] [CrossRef]
- Mester, T.; Field, J.A. Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. J. Biol. Chem. 1998, 273, 15412–15417. [Google Scholar] [CrossRef]
- Pogni, R.; Baratto, C.; Giansanti, S.; Teutloff, C.; Verdin, J.; Valderrrama, B.; Lendzian, F.; Lubits, W.; Vazquez-Duhalt, R.; Basosi, R. Tryptophan-Based radical in the catalytic mechanism of versatile Peroxidase from Bjerkandera adusta. Biochemistry 2005, 44, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Colpa, D.I.; Fraaije, M.W.; Bloois, E. DyP-type peroxidases: A promising and versatile class of enzymes. J. Ind. Microbiol. Biotechnol. 2014, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sugano, Y.; Yoshida, T. DyP-Type Peroxidases: Recent Advances and Perspectives. Int. J. Mol. Sci. 2021, 22, 5556. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ishikawa, K.; Hirai, M.; Shoda, M. Characteristics of a Newly Isolated Fungus, Geotrichum candidum Dec 1, Which Decolorizes Various Dyes. J. Ferment. Bioeng. 1995, 79, 601–607. [Google Scholar] [CrossRef]
- Kim, S.J.; Shoda, M. Purification and Characterization of a Novel Peroxidase from Geotrichum candisum Dec 1 Involved in Decolorization of Dyes. Appl. Environ. Microbiol. 1999, 65, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Johjima, T.; Ohkuma, M.; Kudo, T. Isolation and cDNA Cloning of Novel Hydrogen Peroxide-dependent Phenol Oxidase from the Basidimomycete Termitomyces albuminosus. Appl. Microbiol. Biotechnol. 2003, 61, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Faraco, V.; Piscitelli, A.; Sannia, G.; Giardina, P. Identification of a new member of the dye-decolorizing peroxidase family from Pleurotus ostreatus. World J. Microbiol. Biotechnol. 2007, 23, 889–893. [Google Scholar] [CrossRef]
- Puhse, M.; Szweda, R.T.; Ma, Y.; Jeworrek, C.; Winter, R.; Zorn, H. Marasmius scorodonius extracellular dimeric peroxidase—Exploring its temperature and pressure stability. Biochim. Biophys. Acta 2009, 1794, 1091–1098. [Google Scholar] [CrossRef]
- Liers, C.; Pecyna, M.J.; Kellner, H.; Worrich, A.; Zorn, H.; Steffen, K.T.; Hofrichter, M.; Ullrich, R. Substrate oxidation by dye-decolorizing peroxidases (DyPs) from wood- and litter-degrading agaricomycetes compared to other fungal and plant heme-peroxidases. Appl. Microbiol. Biotechnol. 2013, 97, 5839–5849. [Google Scholar] [CrossRef]
- Salvachua, D.; Prieto, A.; Martinez, A.T.; Martinez, M.J. Characterization of a novel dye-decolorizing peroxidase (DyP)-type enzyme from Irpex lacteus and its application in enzymatic hydrolysis of wheat straw. Appl. Environ. Microbiol. 2013, 79, 4316–4324. [Google Scholar] [CrossRef] [PubMed]
- Kolwek, J.; Behrens, C.; Linke, D.; Krings, U.; Berger, R. Cell-free one-pot conversion of (+)-valencene to (+)-nootkatone by a unique dye-decolorizing peroxidase combined with a laccase from Funalia trogii. J. Ind. Microbial. Biotechnol. 2018, 45, 89–101. [Google Scholar] [CrossRef]
- Krahe, N.K.; Berger, R.G.; Ersoy, F. A DyP-type peroxidase of Pleurotus sapidus with alkene cleaving activity. Molecules 2020, 25, 1536. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Couto, S. Biophotodegradation of pollutants from wastewater. In Bioremediation for Environmental Sustainability; Kumar, V., Saxena, G., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 269–281. ISBN 9780128203187. [Google Scholar] [CrossRef]
- Rathore, S.; Varshney, A.; Mohan, S.; Dahiya, P. An innovative approach of bioremediation in enzymatic degradation of xenobiotics. Biotechnol. Genet. Eng. Rev. 2022, 38, 1–32. [Google Scholar] [CrossRef]
- Lin, S.; Wei, J.; Yang, B.; Zhang, M.; Zhuo, R. Bioremediation of organic pollutants by white rot fungal cytochrome P450: The role and mechanism of CYP450 in biodegradation. Chemosphere 2022, 301, 134776. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent structural insights into cytochrome P450 function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef]
- VandenBrink, B.; Isoherranen, N. The role of metabolites in predicting drug-drug interactions: Focus on irreversible P450 inhibition. Curr. Opin. Drug Discov. 2010, 13, 66. [Google Scholar]
- Cresnar, B.; Petric, S. Cytochrome P450 enzymes in the fungal kingdom. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Young, D.; Rice, J.; Martin, R.; Lindquist, E.; Lipzen, A.; Grigoriev, I.; Hibbett, D. Degradation of bunker C fuel oil by White-rot fungi in sawdust cultures suggests potential applications in bioremediation. PLoS ONE 2015, 10, e0130381. [Google Scholar] [CrossRef]
- Wolfand, J.M.; Lefevre, G.; Luthy, G. Metabolization and degradation kinetics of the urban-use pesticide fipronil by white rot fungus Trametes versicolor. Environ. Sci. Process. Impacts 2016, 18, 1256–1265. [Google Scholar] [CrossRef]
- Mori, T.; Ohono, H.; Ichinose, H.; Kawagishi, H.; Hirai, H. White-rot fungus Phanerochaete chrysosporium metabolizes chloropyridinyl-type neonicotinoid insecticides by an N-dealkylation reaction catalyzed by two cytochrome P450s. J. Hazard. Mater. 2021, 402, 123831. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ohno, H.; Ide, Y.; Ichinose, H.; Mori, T.; Kawagishi, H.; Hirai, H. Identification of the cytochrome P450 involved in the degradation of neonicotinoid insecticide acetamiprid in Phanerochaete chrysosporium. J. Hazard. Mater. 2019, 371, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Marco-Urrea, E.; Perez-Trujillo, M.; Blanquez, P.; Vicent, T.; Caminal, G. Biodegradation of the analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DAD-MS and NMR. Bioresour. Technol. 2010, 101, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Palli, L.; Castellet-Rovira, F.; Perez-Trujillo, M.; Caniani, D.; Sarra-Adroguer, M.; Gori, R. Preliminary evaluation of Pleurotus ostreatus for the removal of selected pharmaceuticals from hospital wastewater. Biotechnol. Prog. 2017, 33, 1529–1537. [Google Scholar] [CrossRef]
- Suhara, H.; Adachi, A.; Kamei, I.; Maekawa, N. Degradation of chlorinated pesticide DDT by litter-decomposing basidiomycetes. Biodegradation 2011, 22, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yamamoto, R.; Yamamoto, Y.; Tokumoto, T.; Dong, J.; Thomas, P.; Hirai, H.; Kawagishi, H. Hydroxylation of bisphenol A by hyper lignin-degrading fungus Phanerochaete sordida YK-624 under non-ligninolytic condition. Chemosphere 2013, 93, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Das, A.; Palaniswamy, M.; Angayarkanni, J. Degradation of benzo a pyrene by Pleurotus ostreatus PO-3 in the presence of defined fungal and bacterial co-cultures. J. Basic Microbiol. 2017, 57, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Liu, C.-X.; Xu, Q.-M.; Cheng, J.-S.; Yuan, Y.-J. Simultaneous removal of ciprofloxacin, norfloxacin, sulfamethoxazole by co-producing oxidative enzymes system of Phanerochaete chrysosporium and Pycnoporus sanguineus. Chemosphere 2018, 195, 146–155. [Google Scholar] [CrossRef]
- Xiao, P.; Kondo, R. Biodegradation and bioconversion of endrin by white rot fungi, Phlebia acanthocystis and Phlebia brevispora. Mycoscience 2019, 60, 255–261. [Google Scholar] [CrossRef]
- Syed, K.; Doddapaneni, H.; Subramanian, V.; Lam, Y.W.; Yadav, J.S. Genome-to-function characterization of novel fungal P450 monooxygenases oxidizing polycyclic aromatic hydrocarbons (PAHs). Biochem. Biophys. Res. Commun. 2010, 399, 492–497. [Google Scholar] [CrossRef]
- Ning, D.; Wang, H. Involvement of cytochrome P450 in pentachlorophenol transformation in a white rot fungus Phanerochaete chrysosporium. PLoS ONE 2012, 7, e45887. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Matsuzaki, F.; Wise, L.; Sakai, Y.; Jindou, S.; Ichinose, H.; Takaya, N.; Kato, M.; Wariishi, H.; Shimizu, M. Biochemical characterization of CYP505D6, a self-sufficient cytochrome P450 from the white-rot fungus Phanerochaete chrysosporium. Appl. Environ. Microbiol. 2018, 84, e01091-18. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Schlosser, D.; Wick, L.Y. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Appl. Ind. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Kuma, A.; Kumar Arora, P. Biotechnological applications of manganese peroxidases for sustainable management. Front. Environ. Sci. 2022, 10, 875157. [Google Scholar] [CrossRef]
- Kasture, N.S. Role of fungi in Metabolism of the nitro-aromatic degradation. In Advances in Agricultural Biotechnology; Mishra, S., Kumar, S., Eds.; Akinik Publications: New Delhi, India, 2023; Volume 8. [Google Scholar]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Appl. Biochem. Biotechnol. 2022, 69, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Zdarta, J.; Jesionowski, T.; Iqbal, H.M. Manganese peroxidases a robust biocatalytic tool-an overview of sources, immobilization, and biotechnological applications. Int. J. Biol. Macromol. 2023, 234, 123531. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rehman, F.; Rafeeq, H.; Waqas, M.; Asghar, A.; Afsheen, N.; Rahdar, A.; Bilal, M.; Iqbal, H.M.N. In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air—A review. Chemosphere 2022, 289, 133252. [Google Scholar] [CrossRef] [PubMed]
- Husain, Q.; Husain, M.; Kulshrestha, Y. Remediation and treatment of organopollutants mediated by peroxidases: A review. Crit. Rev. Biotechnol. 2009, 29, 94–119. [Google Scholar]
- Barber-Zucker, S.; Mindek, V.; García Ruiz, E.; Weinstein, J.J.; Alcalde, M.; Fleishman, J. Stable and functionally diverse versatile peroxidases designed directly from sequences. J. Am. Chem. Soc. 2002, 144, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, J.; Qaria, M.A.; Gao, L.; Zhu, D. Dye Decoloring Peroxidase Structure, Catalytic Properties and Applications: Current Advancement and Futurity. Catalysts 2021, 11, 955. [Google Scholar] [CrossRef]
- Mori, T.; Sugimoto, S.; Ishii, S.; Wu, J.; Nakamura, A.; Dohra, H.; Nagai, K.; Kawagishi, H.; Hirai, H. Biotransformation and detoxification of tetrabromobisphenol A by the white rot fungus Phanerochaete sordida Yk-624. J. Hazard. Mater. 2024, 133469, 4591524. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. A split-face evaluation of a novel pigment-lightening agent compared with no treatment and hydroquinone. J. Am. Acad. Dermatol. 2015, 72, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, M.; Hülsdau, B.; Zelena, K.; Nimtz, M.; de Boer, L.; Berger, R.G.; Zorn, H. Novel peroxidases of Marasmius scorodonius degrade beta-carotene. Appl. Microbiol. Biotechnol. 2008, 77, 1241–1250. [Google Scholar] [CrossRef] [PubMed]
- Noman, E.; Talip, B.A.; Al-Gheethi, A.; Mohamed, R.; Nagao, H. Decolourisation of dyes in greywater by mycoremediation and mycosorption process of fungi from peatland; primary study. Mater. Today Proc. 2020, 31, 23–30. [Google Scholar] [CrossRef]
- Chibueze Azubuike, C.; Chikere, B.; Chijioke, C.; Okpokwasili, G. Biormediation techniques-classification based on site of application: Principles, advantages, limitations and prospects. World J. Microbiol. Biotechnol. 2016, 32, 180. [Google Scholar]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zechal, E.; Hernando Morales, V. Role of fungi in bioremediation of emerging pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahen, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Misra, M. Microplastics in ecosystems. Their implications and mitigations pathways. Environ. Sci. Adv. 2022, 1, 9–29. [Google Scholar] [CrossRef]
- Jaiswal, S.; Singh, D.K.; Shukla, P. Gene editing and systems biology tools for pesticide bioremediation. A review. Front. Microbiol. 2019, 10, 87. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Dye removal by immobilised fungi. Biotechnol. Adv. 2009, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Couto, S. Solid state fermentation for laccases production and their applications. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Ricardo Soccol, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–234. [Google Scholar]
- Otaiku, A.A.; Alhaji, A.I. Fungi consortia in situ biodegradation of xenobiotic, military shooting rangem Kachia, Kaduna, Nigeria. J. Appl. Biotechnol. Bioeng. 2020, 7, 246–274. [Google Scholar]
- Bokade, P.; Purohit, J.; Bajaj, A. Myco-remediation of chlorinated pesticides: Insights into fungal metabolic system. Indian J. Microbiol. 2021, 61, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, H.; Rai, J.P.N.; Jillani, A. Role of fungi in bioremediation of contaminated soil. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Sharma, V.K., Shah, M.P., Parmar, S., Kumar, A., Eds.; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar] [CrossRef]
- Onolame, J.; Falade, A.O.; Aladekoyi, O.J. Applications of microbial laccases in bioremediation of environmental pollutants: Potential issues, challenges, and prospects. In Bioremediation for Environmental Sustainability; Saxena, G., Kumar, V., Shah, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 519–540. ISBN 978012820524. [Google Scholar]
- Galazka, A.; Jankiewicz, U.; Szczepkowski, A. Biochemical characteristics of laccases and their practical application in the removal of xenobiotics from water. Appl. Sci. 2023, 13, 4394. [Google Scholar] [CrossRef]
- Husain, Q. Remediation of phenolic compounds from polluted water by immobilized peroxidases. In Emerging and Eco-Friendly Approaches for Waste Management; Bharagava, R., Chowdahary, P., Eds.; Springer: Singapore, 2019; pp. 329–358. [Google Scholar]
- Ariete-Daronch, N.; Kelbert, M.; Senna Pereira, C.; Hermes de Araujo, P.E.; de Oliveira, D. Elucidating the choice for a precise matrix for laccase immobilization: A review. J. Chem. Eng. 2020, 397, 125506. [Google Scholar] [CrossRef]
- Oliveira-Costa, U.; Cassiano Nascimento, L.F.; Magalahaes Garcia, J.; Neves Monteiro, S.; Santos Luz, F.; Anacleto, P.; García-Filho, F.C. Effect of raphene oxide coating on Natural fiber composite for multilayered ballistic armor. Polymers 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Leal, A.; Poutou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Quevedo-Hidalgo, B.E. A brief history of color, the environmental impact of synthetic dyes and removal by using laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Sun, J.; Fareed, M.F.; Kenawy, E.R.; Ali, S.S. Ecofriendly biodegradation of Reactive Black 5 by newly isolated Sterigmatomyces Halophilus SSA1575, Valued for textile Azo dye wastewater processing and detoxification. Sci. Rep. 2020, 10, 12370. [Google Scholar] [CrossRef]
- Andleeb, S.; Iqbal, Z.; Gukzar, N.; Raza, A.; Ahamad, A. Synthesis, characterization, acute dermal toxicity, anti-inflammatory and wound healing potential of biogenic silver nanaparticles in Balb C. mice. Curr. Pharm. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Lille, F. Valorisation of waste Mussel Shells as Biosorbent for an azo dye elimination. Mater. Sci. Appl. Chem. 2019, 800, 187–192. [Google Scholar]
- Senel, U.; Demirtas, M.; Semel, I.; Dolunay, M.; Guveli, M.E.; Celebi, K.; Denli, S. Investigation of mutagenic effects of synthetic acidic textile dyes by Umu-Test (Salmonella thyphimurium TA1535/pSK1002)—A short term bacterial assay. Int. J. Biol. 2016, 8, 85–91. [Google Scholar] [CrossRef]
- Dai, Y.; Yao, J.; Somg, Y.; Liu, X.; Wang, S.; Yuan, Y. Enhanced performance of immobilized laccase in electrospun fibrous membranes by carbon nanotubes modification and its application for bisphenol A removal from water. J. Hazard. Mater. 2016, 317, 485–493. [Google Scholar] [CrossRef]
- Skariyachan, S.; Manjunatha, V.; Sultana, S.; Jois, C.; Bai, V.; Vasist, K. Novel bacteria consortia isolated from plastic garbage processing areas demonstrated enhanced degradation for low density polyethylene. Environ. Sci. Pollut. Res. Int. 2016, 23, 18307–18319. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Sri Rajendran, D.; Senthil Kumar, P.; Sonai Anand, S.; Vinoth Kumar, V.; Rangasamy, G. Efficient decolorization and detoxification of triarylmethane and azo dyes by porous-cross-linked enzyme aggregates of Pleurotus ostreatus laccase. Chemosphere 2023, 313, 137612. [Google Scholar] [CrossRef]
- Freire Andrade, M.V.; Lima da Silva, K.M.; da Silva Siqueira, J.P.; Pessoa Wanderley, C.R.; Araujo, R.d.S.; Marinho, G.; Rodrigues, K. Azo dye degradation by Phanerochaete chrysosporium in the medium enriched with nitrogen in the presence of primary cosubstrate. Braz. Arch. Biol. Technol. 2013, 56, 867–874. [Google Scholar] [CrossRef]
- Rani, B.; Kumar, V.; Singh, J.; Bisht, S.; Teotia, P.; Sharma, S.; Kela, R. Bioremediation of dyes by fungi isolated from contaminated dye effluent sites for bio-usability. Braz. J. Microbiol. 2014, 45, 1055–1063. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Xu, X.; Jin, L. A Ca-alginate particle co-immobilized with Phanerochaete chrysosporium cells and the combined cross-linked enzyme aggregates from Trametes versicolor. Bioresour. Technol. 2015, 198, 464–469. [Google Scholar] [CrossRef]
- Pessoa Wanderley, C.R.; Vinícius Andrade, M.; José Pereira, L.; Mariho Silva, G.M.; Rodríguez Pessoa, K. Azo dye mineralization by Phanerochaete chrysosporium in a sequencing batch reactor. Braz. Arch. Biol. Technol. 2018, 61. [Google Scholar] [CrossRef]
- Sierra-Solache, R.E.; Muro, C.; Maciel, A.; Illescas, J.; Díaz, M.C.; Carbajal-Franco, G.; Hernández, O.A. Water recovery from textile wastewater treatment by encapsulated cells of Phanerochaete chrysosporium and ultrafiltration system. Biologia 2020, 75, 1717–1729. [Google Scholar] [CrossRef]
- Oliveira Santos, A.D.; Bandeira, L.; Marihno Silva, G.M.; Pessoa Wanderley, C.R.; Rodrigues, K. Use of Phanerochaete chrysosporium in a semibatch reactor for temoval of indigo Carmine from synthetic textile wastewater. Braz. Arch. Biol. Technol. 2021, 64. [Google Scholar] [CrossRef]
- de Almeida, A.P.; Macrae, A.; Ribeiro, B.D.; Nascimento, R.P.D. Decolorization and detoxification of different azo dyes by Phanerochaete chrysosporium ME-446 under submerged fermentation. Braz. J. Microbiol. 2021, 52, 727–738. [Google Scholar] [CrossRef]
- Kües, U.; Nelson, D.; Liu, C.; Yu, G.; Zhang, J.; Li, J. Genome analysis of medicinal Ganoderma spp. with plant-pathogenic and saprotrophic life-styles. Phytochemistry 2015, 114, 18–37. [Google Scholar] [CrossRef]
- Murugesan, K.; Yang, H.; Kim, Y.M.; Jeon, J.R.; Chang, Y.S. Enhanced transformation of malachite green by laccase of Ganoderma lucidum in the presence of natural phenolic compounds. Appl. Microbiol. Biotechnol. 2009, 82, 341–350. [Google Scholar] [CrossRef]
- Manavalan, T.; Manavalan, A.; Kalaichelvan, P.; Thangavelua, P.; Heesed, K. Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem. Eng. J. 2013, 70, 106–114. [Google Scholar] [CrossRef]
- Palazzolo, M.A.; Postemsky, P.D.; Kurina-Sanz, M. From agro-waste to tool: Biotechnological characterization and application of Ganoderma lucidum E47 laccase in dye Decolorization. 3 Biotech 2019, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, S.; Manivasagan, R.; Chinnappan, K. Biodegradation and decolourization of textile dye wastewater using Ganoderma lucidum. 3 Biotech 2013, 3, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhuo, R.; Liu, H.; Yu, D.; Jiang, M.; Zhang, X.; Yang, Y. Efficient Decolorization and detoxification of the sulfonated azo dye Reactive Orange 16 and simulated textile wastewater containing Reactive Orange 16 by the white-rot fungus Ganoderma sp. En3 isolated from the forest of Tzu-chin Mountain in China. Biochem. Eng. J. 2014, 82, 1–9. [Google Scholar] [CrossRef]
- Thaise-Rainert, K.; Alamar Nunes, H.C.; Jefferson Gonçalves, M.; Vieira Helm, C.; Ballod Tavares, L.B. Decolorization of the synthetic dye Remazol Brilliant Blue Reactive (RBBR) by Ganoderma lucidum on bio-adsorbent of the solid bleached sulfate paperboard coated with polyethylene terephthalate. J. Environ. Chem. Eng. 2021, 9, 104990. [Google Scholar] [CrossRef]
- Himanshu; Chamoli, S.; Singh, A.; Kumar Kapoor, R.; Singh, S.; Singh, R.K.; Kumar Saini, J. Purification and characterization of laccse from Ganoderma lucidum and its application in Decolorization of malachite green dye. Bioresour. Technol. Rep. 2023, 21, 101368. [Google Scholar] [CrossRef]
- Torres-Farradá, G.; Manzano León, A.M.; Ramos-Leal, M.; Domínguez, O.; Sánchez-López, M.I.; Vangronsveld, J.; Guerra, G. Biodegradation and detoxification of dyes and industrial effluents by Ganoderma weberianum B-18 immobilized in a lab-scale packed-bed bioreactor. Bioremediat. J. 2018, 22, 20–27. [Google Scholar] [CrossRef]
- Lee, H.; Jang, Y.; Choi, Y.S.; Kim, M.J.; Lee, J.; Lee, H.; Hong, J.S.; Lee, Y.M.; Kim, G.H.; Kim, J.J. Biotechnological procedures to select white rot fungi for the degradation of PHAs. J. Microbiol. Methods 2014, 97, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Peng, L.; Chen, Y.; Zhang, L.; Gu, Z.; Shi, G.; Zhang, K. Production and characterization of thermostable laccase from the mushroom, Ganoderma lucidum, using submerged fermentation. Afr. J. Microbiol. Res. 2012, 6, 1147–1157. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, T.; Cui, B. Biological pretreatment with the white rot fungi and their co-culture to overcome lignocellulosic recalcitrance for improved enzymatic digestion. Bioresources 2014, 9, 3968–3976. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; Amin, H.M.; Shebks, R.I. Modulation of laccase transcriptome during biodegradation of naphthalene by white rot fungus Pleurotus ostreatus. Int. J. Microbiol. 2019, 22, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Embradiri, A.; Katheem Kiyasudeen, S.; Fatemeh Rupani, P.; Hakimi Ibrahim, M. Environmental xenobiotics and its effects on Natural ecosystem. In Plant Responses to Xenobiotics; Singh, A., Ed.; Springer Nature: Singapore, 2016. [Google Scholar] [CrossRef]
- Bonjoko, B. Environmental pharmacology: An overview. In Pharmacology and Therapeutics (Monograph on the Internet); Intech: London, UK, 2014; pp. 133–178. Available online: http://www.intechopen.com/ (accessed on 15 December 2023).
- Marco-Urrea, E.; Perez-Trujillo, M.; Vicent, T.; Caminal, G. Ability of white-rot fungi to remove selected pharmaceuticals and identification of degradation products of ibuprofen by Trametes versicolor. Chemosphere 2009, 74, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Operation of stirred tank reactors (STRs) and fixed-bed reactors (FBRs) with free and immobilized Phanerochaete chrysosporium for the continuous removal of pharmaceutical compounds. Biochem. Eng. J. 2012, 66, 38–45. [Google Scholar] [CrossRef]
- Jaen-Gil, A.; Castellet-Rovira, F.; Llorca, M.; Villagrasa, M.; Sarra, M.; Rodriguez-Mozaz, S.; Barcelo, D. Fungal treatment ofmetoprolol and its recalcitrant metabolite metoprolol acid in hospital wastewater: Biotransformation, sorption and ecotoxicological impact. Water Res. 2019, 152, 171–180. [Google Scholar] [CrossRef]
- Jureczko, M.; Przystas, W.; Krawczyk, T.; Gonciatyz, W.; Rudnicka, K. White-rot fungi-mediated biodegradation of cytostatic grugs-bleomycin and vincristine. J. Hazard. Mater. 2021, 497, 124632. [Google Scholar] [CrossRef]
- Dalecka, B.; Strods, M.; Juhna, T.; Rajarao, G.K. Removal of total phosphorus, ammonia nitrogen and organic carbon from non-sterile municipal wastewater with Trametes versicolor and Aspergillus luchuensis. Microbiol. Res. 2020, 241, 12658. [Google Scholar] [CrossRef]
- Cruz-Morato, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barcelo, D.; Vicent, T.; Sarra, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Lignin peroxidase immobilization on Ca-alginate beads and its dye degradation performance in a packed bed reactor system. Biocatal. Agric. Biotechnol. 2019, 20, 101205. [Google Scholar] [CrossRef]
- Olusegun Bankole, P.; Adeyinka Dekunle, A.; Jeon, B.H.; Prabhhu Govindwar, S. Novel cobiomass degradation of NSAIDs by two wood rot fungi, Ganoderma applanatum and Laetiporus sulphureus: Ligninolytic enzymes induction, isotherm and kinetic studies. Ecotoxicol. Environ. Saf. 2020, 203, 110997. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P.J. Perfluoroalkyl and Polyfluoroalkyl substances in the environment: Terminology, classification, and Origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Daniel Tang, K.; Ayu Kristanti, R. Bioremediation of perfluorochemicals: Current state and the way forward. Bioprocess Biosyst. Eng. 2022, 45, 1093–1109. [Google Scholar] [CrossRef]
- Kissa, E. Fluorinated Surfactants and Repellents, 2nd ed.; Revised and Expanded; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Grgas, D.; Petrina, A.; Štefanac, T.; Bešlo, D.; Landeka Dragičević, T. A Review: Per- and Polyfluoroalkyl Substances—Biological Degradation. Toxics 2023, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Li, L.Y.; Grace, J.R. Review of the fate and transformation of per- and polyfluoroalkyl substances (PFASs) in landfills. Environ. Pollut. 2018, 235, 74–84. [Google Scholar] [CrossRef]
- OECD. Reconciling terminology of the universe of per- and polyfluoroalkyl substances (PFASs). In Recommendations and Practical Guidance (PDF); OECD Environment, Health and Safety Publications Series on Risk Management No. 61; OECD Publishing: Paris, France, 2021; p. 18. [Google Scholar]
- Janousek, R.M.; Lebertz, S.; Knepper, T.P. Previously unidentified sources of perfluoroalkyl substances from building materials and industrial fabrics. Environ. Sci. Process. Impacts 2019, 21, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 2021, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.; Bundschus, M. Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: A review. Environ. Toxicol. Chem. 2014, 33, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Xu, T.; Zhao, D. Treatment of per- and polyfluoroalkyl substances in landfill leachate: Status, chemistry and prospects. Environ. Sci. Water Res. Technol. 2019, 5, 1814–1835. [Google Scholar] [CrossRef]
- Ramhøj, L.; Hass, U.; Boberg, J.; Scholze, M.; Christiansen, S.; Nielsen, F.; Axelstad, M. Perfluorohexane sulfonate (PFHxS) and a mixture of endocrine disrupters reduce thyroxine levels and cause. Toxicol. Sci. 2018, 163, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Kannan, K.; Kajiwara, N.; Costa, M.; Fillmann, G.; Takahashi, S.; Tanabe, S. Perfluorooctanesulfonate and related fluorochemicals in albatrossses, elephant seals, penguins, and polar skuas from the Southern Ocean. Environ. Sci. Technol. 2006, 40, 7642–7648. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, J.C.; Peden-Adams, M.M.; Keller, J.M.; Germolec, D.R. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012, 40, 300–311. [Google Scholar] [CrossRef]
- USEPA US Environmental Protection Agency, 2010/2015 PFOA Stewardship Program. 2006. Available online: https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program (accessed on 10 January 2023).
- ECHA European Chemical Agency. Candidate List of Substances of Very High Concern for Authorization. 2013. Available online: http://echa.europa.eu/web/guest/candidate-list-table (accessed on 14 September 2013).
- Mahinroosta, R.; Senevirathana, L. A review of the emerging treatment technologies for PFAS contaminated soils. J. Environ. Manag. 2020, 255, 109896. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liang, S.; Huang, Q. Laccase induced degradation of perfluorooctanoic acid in a soil slurry. J. Hazard. Mater. 2018, 359, 241–247. [Google Scholar] [CrossRef]
- Tseng, N.; Wang, N.; Szostek, B.; Mahendra, S. Biotransformation of 6:2 fluotelomer alcohol (6:2 FTOH) by a wood-rotting fungus. Environ. Sci. Technol. 2014, 48, 4012–4020. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, J.; Zhang, H.; Wang, Z.; Feng, M.; Chiang, S.-Y.D.; Woodward, D.; Huang, Q. Laccase catalyzed degradation of perfluorooctanoic acid. Environ. Sci. Technol. Lett. 2015, 2, 198–203. [Google Scholar] [CrossRef]
- Tseng, N. Feasibility of Biodegradation of Polyfluoroalkyl and Perfluoroalkyl Substances. Master’s Thesis, UCLA, Los Angeles, CA, USA, 2012. [Google Scholar]
- Merino, N.; Wang, M.; Ambrocio, R.; Mak, K.; O’Connor, E.; Gao, A.; Hawley, E.; Deeb, R.; Tseng, L.; Mahendra, S. Fungal biotransformation of 6:2 fluotelomer alcohol. Remediat. J. 2018, 28, 59–70. [Google Scholar] [CrossRef]
- Bonugli-Santos, R.C.; Dos santos Vasconcelos, M.R.; Passarini, M.R.Z.; Vieira, G.A.L.; Lopes, V.C.P.; Mainardi, P.H.; Dos santos, J.A.; Duarte, L.; Otero, I.V.R.; da silva Yoshida, A.; et al. Marine–derived fungi: Diversity of enzymes and biotechnological applications. Front. Microbiol. 2015, 6, 259. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Farradá, G.; Thijs, S.; Rineau, F.; Guerra, G.; Vangronsveld, J. White Rot Fungi as Tools for the Bioremediation of Xenobiotics: A Review. J. Fungi 2024, 10, 167. https://doi.org/10.3390/jof10030167
Torres-Farradá G, Thijs S, Rineau F, Guerra G, Vangronsveld J. White Rot Fungi as Tools for the Bioremediation of Xenobiotics: A Review. Journal of Fungi. 2024; 10(3):167. https://doi.org/10.3390/jof10030167
Chicago/Turabian StyleTorres-Farradá, Giselle, Sofie Thijs, Francois Rineau, Gilda Guerra, and Jaco Vangronsveld. 2024. "White Rot Fungi as Tools for the Bioremediation of Xenobiotics: A Review" Journal of Fungi 10, no. 3: 167. https://doi.org/10.3390/jof10030167
APA StyleTorres-Farradá, G., Thijs, S., Rineau, F., Guerra, G., & Vangronsveld, J. (2024). White Rot Fungi as Tools for the Bioremediation of Xenobiotics: A Review. Journal of Fungi, 10(3), 167. https://doi.org/10.3390/jof10030167