Integrated Management of the Cotton Charcoal Rot Disease Using Biological Agents and Chemical Pesticides
Abstract
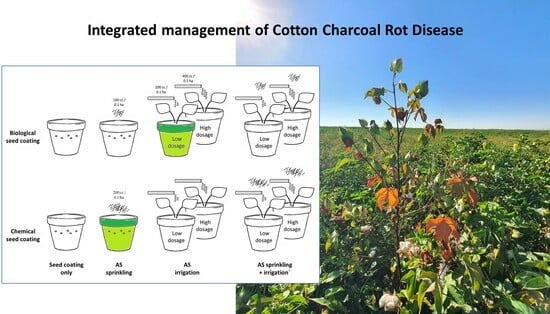
:1. Introduction
2. Materials and Methods
2.1. Fungal Sources and Growth Conditions
2.2. Pot Assay in a Growth Room
2.2.1. The Trial Architecture
2.2.2. The Biological Protective Treatment
2.2.3. The Growth Conditions
2.2.4. Macrophomina phaseolina Infection
2.2.5. Experimental Determinations
2.3. Commercial Field Trial
2.3.1. The Trial Architecture
2.3.2. Growth Protocol and Conditions
2.3.3. Symptoms and Yield Estimation
2.4. Molecular Real-Time PCR Diagnostic
2.4.1. DNA Extraction
2.4.2. qPCR Technique
2.5. Aerial Imaging
2.6. Statistical Analysis
3. Results
3.1. Pot Assay in a Growth Room
3.2. Commercial Field Trial
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarazi, R.; Jimenez, J.L.S.; Vaslin, M.F.S. Biotechnological solutions for major cotton (Gossypium hirsutum) pathogens and pests. Biotechnol. Res. Innov. 2019, 3, 19–26. [Google Scholar] [CrossRef]
- Jans, Y.; von Bloh, W.; Schaphoff, S.; Müller, C. Global cotton production under climate change—Implications for yield and water consumption. Hydrol. Earth Syst. Sci. 2021, 25, 2027–2044. [Google Scholar] [CrossRef]
- Bange, M.P.; Baker, J.T.; Bauer, P.J.; Broughton, K.J.; Constable, G.A.; Luo, Q.; Oosterhuis, D.M.; Osanai, Y.; Payton, P.; Tissue, D.T.; et al. Climate Change and Cotton Production in Modern Farming Systems; CABI: Wallingford, UK, 2016. [Google Scholar]
- Cohen, R.; Elkabetz, M.; Paris, H.S.; Gur, A.; Dai, N.; Rabinovitz, O.; Freeman, S. Occurrence of Macrophomina phaseolina in Israel: Challenges for disease management and crop germplasm enhancement. Plant Dis. 2022, 106, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Suh, S.-O.; Schneider, R.; Russin, J. Host specialization in the charcoal rot fungus, Macrophomina phaseolina. Phytopathology 2001, 91, 120–126. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S.; Abraham, D.; Cohen, R. Interactions between Magnaporthiopsis maydis and Macrophomina phaseolina, the causes of wilt diseases in maize and cotton. Microorganisms 2020, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Kumar, M.; Singh, N.; Chauhan, R.; Mahrashi, A. Evaluation of fungitoxicants and Trichoderma against Macrophomina phaseolina causing root rot of cotton. Plant Dis. Res. 2021, 36, 39–44. [Google Scholar] [CrossRef]
- Degani, O.; Gordani, A.; Dimant, E.; Chen, A.; Rabinovitz, O. The cotton charcoal rot causal agent, Macrophomina phaseolina, biological and chemical control. Front. Plant Sci. 2023, 14, 1272335. [Google Scholar] [CrossRef]
- Islam, M.S.; Haque, M.S.; Islam, M.M.; Emdad, E.M.; Halim, A.; Hossen, Q.M.M.; Hossain, M.Z.; Ahmed, B.; Rahim, S.; Rahman, M.S. Tools to kill: Genome of one of the most destructive plant pathogenic fungi Macrophomina phaseolina. BMC Genom. 2012, 13, 493. [Google Scholar] [CrossRef]
- Abbas, H.K.; Bellaloui, N.; Accinelli, C.; Smith, J.R.; Shier, W.T. Toxin production in soybean (Glycine max L.) plants with charcoal rot disease and by Macrophomina phaseolina, the fungus that causes the disease. Toxins 2019, 11, 645. [Google Scholar] [CrossRef]
- Kumar, S.; Vishnoi, V.K.; Kumar, P.; Dubey, R.C. Survival of Macrophomina phaseolina in plant tissues and soil. In Macrophomina phaseolina; Elsevier: Amsterdam, The Netherlands, 2023; pp. 205–224. [Google Scholar]
- Lodha, S.; Mawar, R. Population dynamics of Macrophomina phaseolina in relation to disease management: A review. J. Phytopathol. 2020, 168, 1–17. [Google Scholar] [CrossRef]
- Basandrai, A.K.; Pandey, A.K.; Somta, P.; Basandrai, D. Macrophomina phaseolina–host interface: Insights into an emerging dry root rot pathogen of mungbean and urdbean, and its mitigation strategies. Plant Pathol 2021, 70, 1263–1275. [Google Scholar] [CrossRef]
- Ghosh, T.; Biswas, M.; Guin, C.; Roy, P. A review on characterization, therapeutic approaches and pathogenesis of Macrophomina phaseolina. Plant Cell Biotechnol. Mol. Biol. 2018, 19, 72–84. [Google Scholar]
- Bashir, M.R.; Mehmood, A.; Sajid, M.; Zeshan, M.A.; Mohsin, M.; Khan, Q.A.; Tahir, F.A. Exploitation of new chemistry fungicides against charcoal rot of sesame caused by Macrophomina phaseolina in Pakistan. Pak. J. Phytopathol. 2017, 29, 257–263. [Google Scholar] [CrossRef]
- Shcherbakova, L. Fungicide resistance of plant pathogenic fungi and their chemosensitization as a tool to increase anti-disease effects of triazoles and strobilurines. Sel’skokhozyaistvennaya Biol. 2019, 54, 875–891. [Google Scholar] [CrossRef]
- Bisen, K.; Singh, V.; Keswani, C.; Ray, S.; Sarma, B.K.; Singh, H. Use of biocontrol agents for the management of seed-borne diseases. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 651–663. [Google Scholar]
- Nakkeeran, S.; Renukadevi, P.; Aiyanathan, K. Exploring the potential of Trichoderma for the management of seed and soil-borne diseases of crops. In Integrated Pest Management of Tropical Vegetable Crops; Springer: Berlin/Heidelberg, Germany, 2016; pp. 77–130. [Google Scholar]
- Degani, O.; Dor, S. Trichoderma biological control to protect sensitive maize hybrids against late wilt disease in the field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.M.; Almeida, N.O.; Côrtes, M.V.d.C.B.; Júnior, M.L.; da Rocha, M.R.; Ulhoa, C.J. Biological control of Pratylenchus brachyurus with isolates of Trichoderma spp. on soybean. Biol. Control 2021, 152, 104425. [Google Scholar] [CrossRef]
- Degani, O.; Gordani, A. New antifungal compound, 6-pentyl-alpha;-pyrone, against the maize late wilt pathogen, Magnaporthiopsis maydis. Agronomy 2022, 12, 2339. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; El-Sayed, A.E.-K.B. Maximizing the efficacy of Trichoderma to control Cephalosporium maydis, causing maize late wilt disease, using freshwater microalgae extracts. Egypt. J. Biol. Pest Control 2018, 28, 48. [Google Scholar] [CrossRef]
- Chaverri, P.; Gazis, R.O.; Samuels, G.J. Trichoderma amazonicum, a new endophytic species on Hevea brasiliensis and H. guianensis from the Amazon basin. Mycologia 2011, 103, 139–151. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate dis 219b promotes growth and delays the onset of the drought response in theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Pozo, M.J.; Cammue, B.P.; Vos, C.M. Belowground defence strategies in plants: The plant–Trichoderma dialogue. In Belowground Defence Strategies in Plants; Springer: Berlin/Heidelberg, Germany, 2016; pp. 301–327. [Google Scholar]
- Howell, C. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Chen, W.; Le Floch, G. Challenges facing the biological control strategies for the management of Fusarium head blight of cereals caused by F. Graminearum. Biol. Control 2017, 113, 26–38. [Google Scholar] [CrossRef]
- Malik, V.K.; Singh, M.; Hooda, K.S.; Yadav, N.K.; Chauhan, P.K. Efficacy of newer molecules, bioagents and botanicals against Maydis leaf blight and banded leaf and sheath blight of maize. Plant Pathol. J. 2018, 34, 121. [Google Scholar] [CrossRef] [PubMed]
- Degani, O.; Becher, P.; Gordani, A. Real-time PCR early detection of Trichoderma treatments efficiency against cotton charcoal rot disease. J. Nat. Pestic. Res. 2023, 4, 100027. [Google Scholar] [CrossRef]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P.A. Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyn, K. A review: Integrated disease management approaches for Macrophomina phaseolina. J. Posit. Sch. Psychol. 2022, 6, 975–988. [Google Scholar]
- Gordani, A.; Hijazi, B.; Dimant, E.; Degani, O. Integrated biological and chemical control against the maize late wilt agent Magnaporthiopsis maydis. Soil Syst. 2023, 7, 1. [Google Scholar] [CrossRef]
- Gal-Hemed, I.; Atanasova, L.; Komon-Zelazowska, M.; Druzhinina, I.S.; Viterbo, A.; Yarden, O. Marine isolates of Trichoderma spp. As potential halotolerant agents of biological control for arid-zone agriculture. Appl. Environ. Microbiol. 2011, 77, 5100–5109. [Google Scholar] [CrossRef]
- Sarejanni, J.; Cortzas, C. The Nature of the Resistance of Cotton to Macrophomina phaseoli (Maubl.) Ashby; CABI: Wallingford, UK, 1936. [Google Scholar]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4325. [Google Scholar] [CrossRef]
- Weller, S.; Elphinstone, J.; Smith, N.; Boonham, N.; Stead, D. Detection of Ralstonia solanacearumstrains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 2000, 66, 2853–2858. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N., Jr. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.K.; Saxena, A.K.; Srivastava, A.K.; Arora, D.K.J.M. Identification and detection of Macrophomina phaseolina by using species-specific oligonucleotide primers and probe. Mycologia 2007, 99, 797–803. [Google Scholar] [CrossRef]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus liberibacter species associated with Citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Orlov-Levin, V.; Meron, M. Applying high-resolution visible-channel aerial imaging of crop canopy to precision irrigation management. Agric. Water Manag. 2019, 216, 196–205. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef]
- Iqbal, U.; Mukhtar, T. Evaluation of biocontrol potential of seven indigenous Trichoderma species against charcoal rot causing fungus, Macrophomina phaseolina. Gesunde Pflanz. 2020, 72, 195–202. [Google Scholar] [CrossRef]
- Iqbal, U.; Mukhtar, T. Inhibitory effects of some fungicides against Macrophomina phaseolina causing charcoal rot. Pak. J. Zool. 2020, 52, 709–715. [Google Scholar] [CrossRef]
- Vashisht, P.; Yadav, N.K.; Kumar, R.; Jangra, P.; Indora, J. Management Strategies for Charcoal Rot of Sesame: A Review. Int. J. Plant Soil Sci. 2023, 35, 2255–2264. [Google Scholar] [CrossRef]
- Das, I.K.; Kishore, B.B.; Srivastava, A.K.; Kumar, S.; Arora, D.K. Ecology, biology and management of Macrophomina phaseolina: An overview. Agric. Important Microorg. 2015, 1, 193–206. [Google Scholar]
- Khalili, E.; Javed, M.A.; Huyop, F.; Rayatpanah, S.; Jamshidi, S.; Wahab, R.A. Evaluation of Trichoderma isolates as potential biological control agent against soybean charcoal rot disease caused by Macrophomina phaseolina. Biotechnol. Biotechnol. Equip. 2016, 30, 479–488. [Google Scholar] [CrossRef]
- Wang, S.-q.; Jia, M.; Meng, W.; Wang, X.-h.; Li, Y.-q.; Jie, C. Combined application of Trichoderma harzianum sh2303 and Difenoconazole-Propiconazolein controlling southern corn leaf blight disease caused by Cochliobolus heterostrophus in maize. J. Integr. Agric. 2019, 18, 2063–2071. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Adrees, H.; Haider, M.S.; Anjum, T.; Akram, W. Inducing systemic resistance in cotton plants against charcoal root rot pathogen using indigenous rhizospheric bacterial strains and chemical elicitors. Crop Prot. 2019, 115, 75–83. [Google Scholar] [CrossRef]
- Bastakoti, S.; Belbase, S.; Manandhar, S.; Arjyal, C. Trichoderma species as biocontrol agent against soil borne fungal pathogens. Nepal J. Biotechnol. 2017, 5, 39–45. [Google Scholar] [CrossRef]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-mediated zno nanoparticles: A green tool for controlling soil-borne pathogens in cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Abdel-Sattar, M.A.; Omar, M.R.; Abd-Elsalam, K.A. Differential antagonism of Trichoderma sp. Against Macrophomina phaseolina. J. Plant Prot. Res. 2007, 47, 91–102. [Google Scholar]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Degani, O.; Weinberg, T.; Graph, S. Chemical control of maize late wilt in the field. Phytoparasitica 2014, 42, 559–570. [Google Scholar] [CrossRef]
- Leadbeater, A. Plant health management: Fungicides and antibiotics. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Castroagudín, V.L.; Ceresini, P.C.; de Oliveira, S.C.; Reges, J.T.; Maciel, J.L.; Bonato, A.L.; Dorigan, A.F.; McDonald, B.A. Resistance to QOI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology 2015, 105, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. Mechanisms of resistance to QOI fungicides in phytopathogenic fungi. Int. Microbiol. 2010, 11, 1–9. [Google Scholar]
- Massi, F.; Torriani, S.F.; Borghi, L.; Toffolatti, S.L. Fungicide resistance evolution and detection in plant pathogens: Plasmopara viticola as a case study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. The QOI Fungicides, the Rise and Fall of a Successful Class of Agricultural Fungicides; InTech: Rijeka, Croatia, 2010. [Google Scholar]
- Koehler, A.; Rogers, L.; Shew, H. Characterization of the cytb gene structure in Macrophomina phaseolina to assess fungicide resistance risk. In Proceedings of the Modern Fungicides and Antifungal Compounds IX, Friedrichroda, Germany, 7–11 April 2019; Deising, H.B., Fraaije, B., Mehl, A., Oerke, E.C., Sierotzki, H., Stammler, G., Eds.; Deutsche Phytomedizinische Gesellschaft e.V. Selbstverlag: Friedrichroda, Germany, 2019; p. 63. [Google Scholar]
- Brent, K.J.; Hollomon, D.W. Fungicide Resistance in Crop Pathogens: How Can It Be Managed? Citeseer: Princeton, NJ, USA, 1995; Volume 1. [Google Scholar]
- Davies, C.R.; Wohlgemuth, F.; Young, T.; Violet, J.; Dickinson, M.; Sanders, J.-W.; Vallieres, C.; Avery, S.V. Evolving challenges and strategies for fungal control in the food supply chain. Fungal Biol. Rev. 2021, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, C. Remote sensing and precision agriculture technologies for crop disease detection and management with a practical application example. Engineering 2020, 6, 528–532. [Google Scholar] [CrossRef]
- Kuswidiyanto, L.W.; Noh, H.-H.; Han, X. Plant disease diagnosis using deep learning based on aerial hyperspectral images: A review. Remote Sens. 2022, 14, 6031. [Google Scholar] [CrossRef]
- Chen, A.; Jacob, M.; Shoshani, G.; Dafny-Yelin, M.; Degani, O.; Rabinovitz, O. Early detection of soil-borne diseases in field crops via remote sensing. In Precision Agriculture’21; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 37–44. [Google Scholar]
- Ortiz-Bustos, C.M.; Testi, L.; García-Carneros, A.B.; Molinero-Ruiz, L. Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. Eur. J. Plant Pathol. 2015, 144, 383–397. [Google Scholar] [CrossRef]
- Degani, O.; Chen, A.; Dor, S.; Orlov-Levin, V.; Jacob, M.; Shoshani, G.; Rabinovitz, O. Remote evaluation of maize cultivars susceptibility to late wilt disease caused by Magnaporthiopsis maydis. J. Plant Pathol. 2022, 104, 509–525. [Google Scholar] [CrossRef]
- Fiaz, S.; Khan, S.A.; Younas, A.; Shahzad, K.; Ali, H.; Noor, M.A.; Ashraf, U.; Nadeem, F. Application of CRISPR/CAS system for genome editing in cotton. In Crispr and RNAi Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 277–301. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D. Botanical pesticides for eco-friendly pest management: Drawbacks and limitations. In Pesticides in Crop Production: Physiological and Biochemical Action; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020; pp. 181–193. [Google Scholar]








| Species | Designation | Origin | Reference |
|---|---|---|---|
| Trichoderma sp. O.Y. 7107 | T7107 | Psammocinia sp. 1 | [30,34] |
| Trichoderma longibrachiatum | T7407 | Psammocinia sp. 1 | [20,30,34] |
| Trichoderma asperellum | P1 | Zea mays, Prelude cv. | [14,30] |
| No. | Treatment | Designation | Azoxystrobin Dosage (Active Ingredient) | Timetable |
|---|---|---|---|---|
| 1 | Chemical seed coating 1 | SC only | Non (Control) | Before seeding |
| 2 | Biological seed coating with Trichoderma species mix 2 | |||
| 3 | Chemical seed coating and AS 3 sprinkling in the sowing hole. | Sp | 0.88 mg dissolved in 20 mL DDW 4 (equivalent to 200 mL/0.1 ha) | With the seeding |
| 4 | Biological seed coating and AS sprinkling in the sowing hole. | 0.44 mg dissolved in 20 mL DDW (equivalent to 100 mL/0.1 ha) | ||
| 5 | Chemical seed coating and AS irrigation (low dosage) | D200 | 0.88 mg dissolved in 10 mL DDW | 10- and 21-days post sowing |
| 6 | Biological seed coating and AS irrigation (low dosage) | |||
| 7 | Chemical seed coating and AS irrigation (high dosage) | D400 | 1.76 mg dissolved in 10 mL DDW (equivalent to 400 mL/0.1 ha) | |
| 8 | Biological seed coating and AS irrigation (high dosage) | |||
| 9 | Chemical seed coating, AS sprinkling, and irrigation (low dosage) | Sp + D200 | As in treatments 3–4 + 5–6 | With the seeding and 10- and 21-day post sowing |
| 10 | Biological seed coating, AS sprinkling, and irrigation (low dosage) | |||
| 11 | Chemical seed coating, AS sprinkling, and irrigation (high dosage) | Sp + D400 | As in treatments 3–4 + 7–8 | |
| 12 | Biological seed coating, AS sprinkling, and irrigation (high dosage) |
| Date | Inoculation and Sowing | Days from Sowing |
| 7 May 2023 | 1st inoculation (sterilized infected millet grains) | −7 |
| 14 May 2023 | Seeding and pesticide (Azoxystrobin) sprinkling | 0 |
| 21 May 2023 | 2nd inoculation (3 discs/sprout) | 7 |
| 28 May 2023 | 3rd inoculation (3 discs/sprout) | 14 |
| Date | Pesticide Irrigation Treatments and Above-Ground Sprouting Assessment | Days from Sowing |
| 24 May 2023 | Pesticide I application and soil surface peek evaluation | 10 |
| 4 June 2023 | Pesticide II application (11 days from Pesticide I) | 21 |
| Date | Sampling | Days from Sowing |
| 24 May 2023 | Above-ground emergence estimation | 10 |
| 12 June 2023 | Mid-experiment sampling and thinning | 29 |
| 5 July 2023 | Final sampling | 52 |
| Date | Seeding and Sprouting Assessment | Days from Sowing |
|---|---|---|
| 9 April 2023 | Seeding | 0 |
| 23 April 2023 | Soil surface peek valuation | 14 |
| Irrigation and Azoxystrobin treatments | ||
| 22 May 2023 | Watering opening | 43 |
| 23 May 2023 | 1st Azoxystrobin irrigation | 44 |
| 26 June 2023 | 2nd Azoxystrobin irrigation | 78 |
| Sampling and harvest | ||
| 22 June 2023 | Midseason sampling | 74 |
| 7 September 2023 | End season sampling | 151 |
| 18 September 2023 | Remote sensing (visible and thermal imaging) | 162 |
| 5 November 2023 | Harvest and yield assessment | 210 |
| Pairs | Primer | Sequence | Uses | Amplification | References |
|---|---|---|---|---|---|
| Pair 1 | MpKFI MpKRI | 5′-CCGCCAGAGGACTATCAAAC-3′ 5′-CGTCCGAAGCGAGGTGTATT-3′ | Target gene | 300–400 bp M. phaseolina species-specific fragment | [40] |
| Pair 2 | COX-F COX-R | 5′-GTATGCCACGTCGCATTCCAGA-3′ 5′-CAACTACGGATATATAAGRRCCRRAACTG-3′ | Control | Cytochrome c oxidase (COX) gene product | [37,41] |
| GRVI Index | Thermal Imaging | ||||||
|---|---|---|---|---|---|---|---|
| Treatment | Mean | SE | % | Mean | SE | % | |
| Chemical SC 2 | SC only | 0.0928 | 0.0548 | 100% | 35.33 | 1.277 | 100.00% |
| Sp | 0.0951 | 0.056 | 103% | 34.96 | 1.212 | 98.90% | |
| D200 | 0.0929 | 0.0531 | 100% | 35.69 | 1.288 | 101.00% | |
| D400 | 0.0912 | 0.0532 | 98% | 35.36 | 1.259 | 100.10% | |
| Sp + D200 | 0.0961 | 0.0535 | 104% | 35.29 | 1.246 | 99.90% | |
| Sp + D400 | 0.0932 | 0.0554 | 100% | 35.27 | 1.347 | 99.80% | |
| Biological SC | SC only | 0.0922 | 0.0547 | 99% | 35.19 | 1.234 | 99.60% |
| Sp | 0.0899 | 0.0517 | 97% | 35.63 | 1.269 | 100.80% | |
| D200 | 0.0951 | 0.0565 | 102% | 35.54 | 1.342 | 100.60% | |
| D400 | 0.0926 | 0.0538 | 100% | 35.23 | 1.265 | 99.70% | |
| Sp + D200 | 0.0917 | 0.0519 | 99% | 35.59 | 1.318 | 100.70% | |
| Sp + D400 | 0.0943 | 0.0553 | 102% | 35.1 | 1.297 | 99.30% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degani, O.; Chen, A.; Dimant, E.; Gordani, A.; Malul, T.; Rabinovitz, O. Integrated Management of the Cotton Charcoal Rot Disease Using Biological Agents and Chemical Pesticides. J. Fungi 2024, 10, 250. https://doi.org/10.3390/jof10040250
Degani O, Chen A, Dimant E, Gordani A, Malul T, Rabinovitz O. Integrated Management of the Cotton Charcoal Rot Disease Using Biological Agents and Chemical Pesticides. Journal of Fungi. 2024; 10(4):250. https://doi.org/10.3390/jof10040250
Chicago/Turabian StyleDegani, Ofir, Assaf Chen, Elhanan Dimant, Asaf Gordani, Tamir Malul, and Onn Rabinovitz. 2024. "Integrated Management of the Cotton Charcoal Rot Disease Using Biological Agents and Chemical Pesticides" Journal of Fungi 10, no. 4: 250. https://doi.org/10.3390/jof10040250
APA StyleDegani, O., Chen, A., Dimant, E., Gordani, A., Malul, T., & Rabinovitz, O. (2024). Integrated Management of the Cotton Charcoal Rot Disease Using Biological Agents and Chemical Pesticides. Journal of Fungi, 10(4), 250. https://doi.org/10.3390/jof10040250