Analysis of Transposable Elements in Coccidioides Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genomes
2.2. Analysis tools
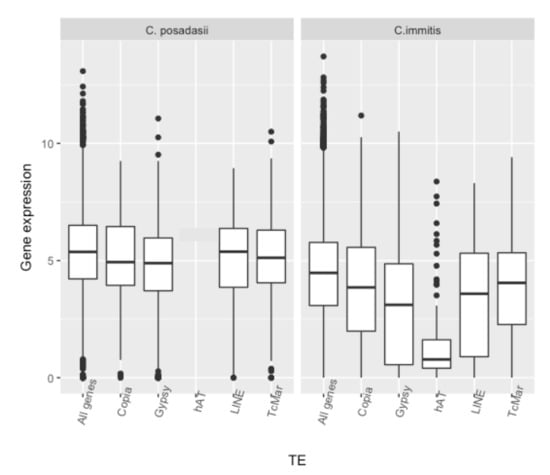
3. Results
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Teixeira, M.M.; Barker, B.M. Use of Population Genetics to Assess the Ecology, Evolution, and Population Structure of Coccidioides. Emerg. Infect. Dis. 2016, 22, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Koenig, G.; White, T.J.; Taylor, J.W. A test for concordance between the multilocus genealogies of genes and microsatellites in the pathogenic fungus Coccidioides immitis. Mol. Biol. Evol. 2000, 17, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Neafsey, D.E.; Barker, B.M.; Sharpton, T.J.; Park, D.J.; Whiston, E.; Hung, C.-Y.; McMahan, C.; White, J.; Sykes, S.; Heiman, D.; et al. Population genomic sequencing of Coccidioides Fungi reveals recent hybridization and transposon control. Genome Res. 2010, 20, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.A.; Tarcha, E.J.; Cole, G.T.; Inglis, D.O.; Sil, A.; Heitman, J. Evolution of the mating type locus: Insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryot. Cell 2007, 6, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Mandel, M.A.; Barker, B.M.; Kroken, S.; Rounsley, S.D.; Orbach, M.J. Genomic and population analyses of the mating type loci in Coccidioides species reveal evidence for sexual reproduction and gene acquisition. Eukaryot. Cell 2007, 6, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N.; Fierer, J. Coccidioidomycosis: A reemerging infectious disease. Emerg. Infect. Dis. 1996, 2, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, L.; Nix, D.; Wright, M.; Lindberg, E.; Fagan, T.; Lieberman, D.; Stoffer, T.; Ampel, N.M.; Galgiani, J.N. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg. Infect. Dis. 2006, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Barker, B.M.; Hoover, S.; Nix, D.E.; Ampel, N.M.; Frelinger, J.A.; Orbach, M.J.; Galgiani, J.N. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin. Microbiol. Rev. 2013, 26, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Whiston, E.; Zhang Wise, H.; Sharpton, T.J.; Jui, G.; Cole, G.T.; Taylor, J.W. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS ONE 2012, 7, e41034. [Google Scholar] [CrossRef] [PubMed]
- Converse, J.L. Effect of physico-chemical environment of spherulation of Coccidioides immitis in a chemically defined medium. J. Bacteriol. 1956, 72, 784–792. [Google Scholar] [PubMed]
- Viriyakosol, S.; Singhania, A.; Fierer, J.; Goldberg, J.; Kirkland, T.N.; Woelk, C.H. Gene expression in human fungal pathogen Coccidioides immitis changes as arthroconidia differentiate into spherules and mature. BMC Microbiol. 2013, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N. A few shared up-regulated genes may influence conidia to yeast transformation in dimorphic fungal pathogens: Table 1. Med. Mycol. 2016, 54, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Boyce, K.J.; McLauchlan, A.; Schreider, L.; Andrianopoulos, A. Intracellular Growth Is Dependent onTyrosine Catabolism in the Dimorphic FungalPathogen Penicillium marneffei. PLoS Pathog. 2015, 11, e1004790. [Google Scholar] [CrossRef] [PubMed]
- Fedoroff, N.V. Presidential address. Transposable elements, epigenetics, and genome evolution. Science 2012, 338, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Feschotte, C.; Pritham, E.J. DNA Transposons and the Evolution of Eukaryotic Genomes. Annu. Rev. Genet. 2007, 41, 331–368. [Google Scholar] [CrossRef] [PubMed]
- Rebollo, R.; Romanish, M.T.; Mager, D.L. Transposable Elements: An Abundant and Natural Source of Regulatory Sequences for Host Genes. Annu. Rev. Genet. 2012, 46, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Leem, Y.-E.; Levin, H.L. Transposon integration enhances expression of stress response genes. Nucleic Acids Res. 2012, 41, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Casacuberta, E.; González, J. The impact of transposable elements in environmental adaptation. Mol. Ecol. 2013, 22, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Biemont, C. A Brief History of the Status of Transposable Elements: From Junk DNA to Major Players in Evolution. Genetics 2010, 186, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Kempken, F.; Kück, U. Transposons in filamentous Fungi—Facts and perspectives. Bioessays 1998, 20, 652–659. [Google Scholar] [CrossRef]
- Parlange, F.; Oberhaensli, S.; Breen, J.; Platzer, M.; Taudien, S.; Šimková, H.; Wicker, T.; Doležel, J.; Keller, B. A major invasion of transposable elements accounts for the large size of the Blumeria graminis f.sp. tritici genome. Funct. Integr. Genom. 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seberg, O.; Petersen, G. A unified classification system for eukaryotic transposable elements should reflect their phylogeny. Nat. Rev. Genet. 2009, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-W.; Wessler, S.R. The catalytic domain of all eukaryotic cut-and-paste transposase superfamilies. Proc. Natl. Acad. Sci. USA 2011, 108, 7884–7889. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.F.; Silva, J.C.; Mizubuti, E.S.; Araújo, E.F.; Condon, B.J.; Turgeon, B.; Queiroz, M.V. Characterization and potential evolutionary impact of transposable elements in the genome of Cochliobolus heterostrophus. BMC Genom. 2014, 15, 536. [Google Scholar] [CrossRef] [PubMed]
- Muszewska, A.; Hoffman-Sommer, M.; Grynberg, M. LTR Retrotransposons in Fungi. PLoS ONE 2011, 6, e29425. [Google Scholar] [CrossRef] [PubMed]
- Daboussi, M.-J.; Capy, P. Transposable Elements in Filamentous Fungi. Annu. Rev. Microbiol. 2003, 57, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Grandaubert, J.; Balesdent, M.-H.; Rouxel, T. Evolutionary and Adaptive Role of Transposable Elements in Fungal Genomes, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 70, pp. 79–107. [Google Scholar]
- Atkinson, P.W. hAT Transposable Elements. Microbiol. Spectr. 2015, 3, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Sharpton, T.J.; Rounsley, S.D.; Gardner, M.J.; Wortman, J.R.; Jordar, V.S.; Maiti, R.; Kodira, C.D.; Neafsey, D.E.; Zeng, Q.; Hung, C.-Y.; et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009, 19, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Brunk, B.P.; Brestelli, J.; Fischer, S.; Harb, O.S.; Kissinger, J.C.; Li, W.; Nayak, V.; Pinney, D.F.; Stoeckert, C.J.; et al. FungiDB: An integrated functional genomics database for Fungi. Nucleic Acids Res. 2012, 40, D675–D681. [Google Scholar]
- FingiDB. Available online: http://fungidb.org/fungidb/ (accessed on 10 January 2017).
- RepeatMasker. Available online: http://www.repeatmasker.org/ (accessed on 6 January 2017).
- Castanera, R.; López-Varas, L.; Borgognone, A.; LaButti, K.; Lapidus, A.; Schmutz, J.; Grimwood, J.; Pérez, G.; Pisabarro, A.G.; Grigoriev, I.V.; et al. Transposable Elements versus the Fungal Genome: Impact on Whole-Genome Architecture and Transcriptional Profiles. PLoS Genet. 2016, 12, e1006108. [Google Scholar] [CrossRef] [PubMed]
- GitHub. Available online: https://github.com/hyphaltip/cocci_repeats/ (accessed on 10 January 2017).
- Galaxy. Available online: https://usegalaxy.org/ (accessed on 9 January 2017).
- iTOL. Available online: https://itol.embl.de/ (accessed on 7 January 2017).
- Integrative Genomics Viewer. Available online: http://software.broadinstitute.org/software/igv/userguide (accessed on 12 October 2017).
- Clutterbuck, A.J. Genomic evidence of repeat-induced point mutation (RIP) in filamentous ascomycetes. Fungal Genet. Biol. 2011, 48, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Baucom, R.S.; Estill, J.C.; Chaparro, C.; Upshaw, N.; Jogi, A.; Deragon, J.-M.; Westerman, R.P.; SanMiguel, P.J.; Bennetzen, J.L. Exceptional Diversity, Non-Random Distribution, and Rapid Evolution of Retroelements in the B73 Maize Genome. PLoS Genet. 2009, 5, e1000732. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, B.; Gill, N.; Hamelin, R.C.; Goodwin, S.B. The landscape of transposable elements in the finished genome of the fungal wheat pathogen Mycosphaerella graminicola. BMC Genom. 2014, 15, 1132–1149. [Google Scholar] [CrossRef] [PubMed]
- Labbé, J.; Murat, C.; Morin, E.; Tuskan, G.A.; Le Tacon, F.; Martin, F. Characterization of Transposable Elements in the Ectomycorrhizal Fungus Laccaria bicolor. PLoS ONE 2012, 7, e40197. [Google Scholar] [CrossRef] [PubMed]
- Yadav, C.B.; Bonthala, V.S.; Muthamilarasan, M.; Pandey, G.; Khan, Y.; Prasad, M. Genome-wide development of transposable elements-based markers in foxtail millet and construction of an integrated database. DNA Res. 2015, 22, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Loftus, B.J.; Fung, E.; Roncaglia, P.; Rowley, D.; Amedeo, P.; Bruno, D.; Vamathevan, J.; Miranda, M.; Anderson, I.J.; Fraser, J.A.; et al. The genome of the basidiomycetous yeast and human pathogen Cryptococcus neoformans. Science 2005, 307, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.E.; Boeke, J.D. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 1996, 10, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, R.A.; Lucas, B.A.; Maquat, L.E. Retrotransposons as regulators of gene expression. Science 2016, 351, aac7247. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Weigel, D.; Smith, L.M. Transposon Variants and Their Effects on Gene Expression in Arabidopsis. PLoS Genet. 2013, 9, e1003255. [Google Scholar] [CrossRef] [PubMed]
- Chuong, E.B.; Elde, N.C.; Feschotte, C. Regulatory activities of transposable elements: From conflicts to benefits. Nat. Rev. Genet. 2017, 18, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Makarevitch, I.; Waters, A.J.; West, P.T.; Stitzer, M.; Hirsch, C.N.; Ross-Ibarra, J.; Springer, N.M. Transposable Elements Contribute to Activation of Maize Genes in Response to Abiotic Stress. PLoS Genet. 2015, 11, e1004915. [Google Scholar] [CrossRef] [PubMed]
- Cowley, M.; Oakey, R.J. Transposable elements re-wire and fine-tune the transcriptome. PLoS Genet. 2013, 9, e1003234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yazaki, J.; Sundaresan, A.; Cokus, S.; Chan, S.W.L.; Chen, H.; Henderson, I.R.; Shinn, P.; Pellegrini, M.; Jacobsen, S.E.; et al. Genome-wide High-Resolution Mapping and Functional Analysis of DNA Methylation in Arabidopsis. Cell 2006, 126, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Aramayo, R.; Selker, E.U. Neurospora crassa, a Model System for Epigenetics Research. Cold Spring Harb. Perspect. Biol. 2013, 5, a017921. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; He, K.; Ma, Y.; Su, N.; He, H.; Stolc, V.; Tongprasit, W.; Jin, W.; Jiang, J.; et al. High-Resolution Mapping of Epigenetic Modifications of the Rice Genome Uncovers Interplay between DNA Methylation, Histone Methylation, and Gene Expression. Plant Cell Online 2008, 20, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Lippman, Z.; Gendrel, A.-V.; Black, M.; Vaughn, M.W.; Dedhia, N.; McCombie, W.R.; Lavine, K.; Mittal, V.; May, B.; Kasschau, K.D.; et al. Role of transposable elements in heterochromatin and epigenetic control. Nature 2004, 430, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, F.E.; Garre, V. RNA Interference in Fungi: Retention and Loss. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Zhu, Y.; Dai, J.; Fuerst, P.G.; Voytas, D.F. Controlling integration specificity of a yeast retrotransposon. Proc. Natl. Acad. Sci. USA 2003, 100, 5891–5895. [Google Scholar] [CrossRef] [PubMed]
- Hua-Van, A.; Le Rouzic, A.; Maisonhaute, C.; Capy, P. Abundance, distribution and dynamics of retrotransposable elements and transposons: Similarities and differences. Cytogenet. Genome Res. 2005, 110, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Miyao, A. Target Site Specificity of the Tos17 Retrotransposon Shows a Preference for Insertion within Genes and against Insertion in Retrotransposon-Rich Regions of the Genome. Plant Cell Online 2003, 15, 1771–1780. [Google Scholar] [CrossRef]





| C. immitis | C. posadasii | |||||||
|---|---|---|---|---|---|---|---|---|
| Type | Number | Mean Length | ORFs > 300 nt (%) | GC Content (%) | Number | Mean length | ORF > 300 nt (%) | GC Content (%) |
| DNA/TcMar | 286 | 884 | 113 (40) | 37 | 575 | 991 | 456 (79) | 36 |
| -Ant1 | 63 | 31 | ||||||
| -Fot1 | 134 | 430 | ||||||
| -Pogo | 27 | 29 | ||||||
| -Tc1 | 66 | 83 | ||||||
| DNA/hAT | 100 | 2416 | 80 (80) | 36 | 37 | 1232 | 15 (40) | 31 |
| LTR/Gypsy | 1204 | 2089 | 1557 (130) | 33 | 1199 | 2046 | 1387 (116) | 34 |
| LTR/Copia | 287 | 1351 | 151 (52) | 38 | 190 | 937 | 131 (69) | 39 |
| LINE | 364 | 1331 | 239 (66) | 28 | 225 | 1237 | 144 (64) | 31 |
| C. immitis | C. posadasii | |||||
|---|---|---|---|---|---|---|
| Total | Pol Domain | None | Total | Pol Domain | None | |
| Number of TEs | 1204 | 338 (28%) | 866 (72%) | 1199 | 260 (22%) | 938 (78%) |
| Associated loci | 571 | 60 (11%) | 511 (89%) | 341 | 24 (7%) | 317 (91%) |
| Number of TEs | |||||
|---|---|---|---|---|---|
| C. posadasii orthologs | ≥4 | 3 | 2 | 1 | None |
| 27 | 17 | 2 | 5 | 4 | 1 |
| Control | Upstream a | Overlap b | Downstream c | ||||
|---|---|---|---|---|---|---|---|
| Mean | Number | Mean | Number | Mean | Number | Mean | |
| All genes | 4.032 | 992 | 773 | ||||
| TcMar | 115 | 3.734 | 131 | 3.759 | 115 | 3.734 | |
| hAT | 75 | 1.134 | 73 | 1.135 | 77 | 1.292 | |
| Copia | 119 | 3.242 | 69 | 3.343 | 137 | 4.229 | |
| Gypsy | 272 | 3.027 | 217 | 2.001 | 312 | 2.156 | |
| C. immitisb | C. posadasii | ||||
|---|---|---|---|---|---|
| ID | Name | Odds Ratio | p (Bonferroni) | Odds Ratio | p (Bonferroni) |
| GO:0016310 | Phosphorylation | 3.35 | 1.43 × 10−7 | 3.34 | 7.35 × 10−7 |
| GO:0006468 | protein phosphorylation | 3.47 | 1.56 × 10−7 | 3.60 | 2.10 × 10−6 |
| GO:0036211 | protein modification process | 2.72 | 3.87 × 10−6 | 2.48 | 2.90 × 10−4 |
| GO:0006464 | cellular protein modification process | 2.72 | 3.87 × 10−6 | 2.48 | 2.90 × 10−4 |
| GO:0006796 | phosphate-containing compound metabolic process | 2.45 | 1.17 × 10−5 | 2.07 | 4.41 × 10−3 |
| GO:0006793 | phosphorus metabolic process | 2.43 | 1.36 × 10−5 | 2.05 | 5.00 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkland, T.N.; Muszewska, A.; Stajich, J.E. Analysis of Transposable Elements in Coccidioides Species. J. Fungi 2018, 4, 13. https://doi.org/10.3390/jof4010013
Kirkland TN, Muszewska A, Stajich JE. Analysis of Transposable Elements in Coccidioides Species. Journal of Fungi. 2018; 4(1):13. https://doi.org/10.3390/jof4010013
Chicago/Turabian StyleKirkland, Theo N., Anna Muszewska, and Jason E. Stajich. 2018. "Analysis of Transposable Elements in Coccidioides Species" Journal of Fungi 4, no. 1: 13. https://doi.org/10.3390/jof4010013
APA StyleKirkland, T. N., Muszewska, A., & Stajich, J. E. (2018). Analysis of Transposable Elements in Coccidioides Species. Journal of Fungi, 4(1), 13. https://doi.org/10.3390/jof4010013