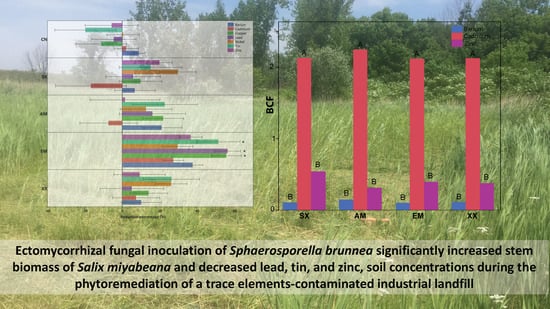
Ectomycorrhizal Fungal Inoculation of Sphaerosporella brunnea Significantly Increased Stem Biomass of Salix miyabeana and Decreased Lead, Tin, and Zinc, Soil Concentrations during the Phytoremediation of an Industrial Landfill
Abstract
:1. Introduction
2. Materials and Method
2.1. The Experimental Site
2.2. Experimental Design and Biological Material
2.3. Rhizophagus irregularis
2.4. Sphaerosporella brunnea
2.5. Sampling and Plant Measures
2.6. TE Concentrations
2.7. Statistical Analyses
3. Results
Salix myabeana Showed High Survival Rates and Cd Extraction Efficiency, but Only the EM Fungi Treatment Showed Significant Effect
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cunningham, S.D.; Berti, W.R.; Huang, J.W. Phytoremediation of contaminated soils. Trends Biotechnol. 1995, 13, 393–397. [Google Scholar] [CrossRef]
- Navarro, M.C.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochem. Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Garbisu, C.; Alkorta, I. Phytoextraction: A cost-effective plant-based technology for the removal of metals from the environment. Bioresour. Technol. 2001, 77, 229–236. [Google Scholar] [CrossRef]
- Giller, K.E.; Witter, E.; McGrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on Remediation Technologies of Soil Contaminated by Heavy Metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [Green Version]
- Marchand, C.; Mench, M.; Jani, Y.; Kaczala, F.; Notini, P.; Hijri, M.; Hogland, W. Pilot scale aided-phytoremediation of a co-contaminated soil. Sci. Total Environ. 2018, 618, 753–764. [Google Scholar] [CrossRef]
- Robichaud, K.; Girard, C.; Dagher, D.; Stewart, K.; Labrecque, M.; Hijri, M.; Amyot, M. Local fungi, willow and municipal compost effectively remediate petroleum-contaminated soil in the Canadian North. Chemosphere 2019, 220, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Robichaud, K.; Stewart, K.; Labrecque, M.; Hijri, M.; Cherewyk, J.; Amyot, M. An ecological microsystem to treat waste oil contaminated soil: Using phytoremediation assisted by fungi and local compost, on a mixed-contaminant site, in a cold climate. Sci. Total Environ. 2019, 672, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Camelia, V.; Monica, M.; Leonard, M.-C.; Anca, M.-C.; Lucia, M. Evaluation of the phytoremediation potential of the Salix caprea in tailing ponds. Analele Universitatii din Oradea Fascicula Biologie 2009, TOM XVi, 141–149. [Google Scholar]
- Laidlaw, W.S.; Arndt, S.K.; Huynh, T.T.; Gregory, D.; Baker, A.J. Phytoextraction of heavy metals by willows growing in biosolids under field conditions. J. Environ. Qual. 2012, 41, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Sinhal, V.K.; Srivastava, A.; Singh, V.P. EDTA and citric acid mediated phytoextraction of Zn, Cu, Pb and Cd through marigold (Tagetes erecta). J. Environ. Biol./Acad. Environ. Biol. India 2010, 31, 255–259. [Google Scholar]
- French, C.J.; Dickinson, N.M.; Putwain, P.D. Woody biomass phytoremediation of contaminated brownfield land. Environ. Pollut. 2006, 141, 387–395. [Google Scholar] [CrossRef]
- Laureysens, I.; Blust, R.; De Temmerman, L.; Lemmens, C.; Ceulemans, R. Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture: I. Seasonal variation in leaf, wood and bark concentrations. Environ. Pollut. 2004, 131, 485–494. [Google Scholar] [CrossRef]
- Laureysens, I.; De Temmerman, L.; Hastir, T.; Van Gysel, M.; Ceulemans, R. Clonal variation in heavy metal accumulation and biomass production in a poplar coppice culture. II. Vertical distribution and phytoextraction potential. Environ. Pollut. 2005, 133, 541–551. [Google Scholar] [CrossRef]
- Tőzsér, D.; Magura, T.; Simon, E. Heavy metal uptake by plant parts of willow species: A meta-analysis. J. Hazard. Mater. 2017, 336, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Hrynkiewicz, K.; Baum, C. Selection of ectomycorrhizal willow genotype in phytoextraction of heavy metals. Environ. Technol. 2013, 34, 225–230. [Google Scholar] [CrossRef]
- Pulford, I. Phytoremediation of heavy metal-contaminated land by trees—A review. Environ. Int. 2003, 29, 529–540. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Weih, M.; Romero, M.A.; Charles, J.; Hust, S.; McIvor, I.; Karp, A.; Trybush, S.; Labrecque, M.; Teodorescu, T.I. Salix: Botany and global horticulture. Hortic. Rev. 2007, 34, 447–489. [Google Scholar]
- Argus, G.W. Infrageneric Classification of Salix (Salicaceae) in the New World; Systematic Botany Monographs; American Society of Plant Taxonomists: Ann Arbor, MI, USA, 1997; pp. 1–121. [Google Scholar]
- Pray, T.J. The Effect of Mycorrhizal Fungi Associated with Willows Growing on Marginal Agricultural Land. Ph.D. Thesis, Université de Montréal, Montréal, QC, Canada, March 2018. Available online: https://papyrus.bib.umontreal.ca/xmlui/handle/1866/20593 (accessed on 18 June 2018).
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Access Online via Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wu, Q.-S.; Xia, R.-X.; Zou, Y.-N. Improved soil structure and citrus growth after inoculation with three arbuscular mycorrhizal fungi under drought stress. Eur. J. Soil Biol. 2008, 44, 122–128. [Google Scholar] [CrossRef]
- Glassman, S.I.; Casper, B.B. Biotic contexts alter metal sequestration and AMF effects on plant growth in soils polluted with heavy metals. Ecology 2012, 93, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, S.E.; Hijri, M.; St-Arnaud, M. Effect of arbuscular mycorrhizal fungi on trace metal uptake by sunflower plants grown on cadmium contaminated soil. New Biotechnol. 2013, 30, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]
- Mrnka, L.; Kuchár, M.; Cieslarová, Z.; Matějka, P.; Száková, J.; Tlustoš, P.; Vosátka, M. Effects of Endo- and Ectomycorrhizal Fungi on Physiological Parameters and Heavy Metals Accumulation of Two Species from the Family Salicaceae. Water Air Soil Pollut. 2012, 223, 399–410. [Google Scholar] [CrossRef]
- Turnau, K.; Orlowska, E.; Ryszka, P.; Zubek, S.; Anielska, T.; Gawronski, S.; Jurkiewicz, A. Role of mycorrhizal fungi in phytoremediation and toxicity monitoring of heavy metal rich industrial wastes in southern Poland. In Soil and Water Pollution Monitoring, Protection and Remediation; Twardowska, I., Allen, H., Häggblom, M., Stefaniak, S., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 69, pp. 533–551. [Google Scholar]
- Bécard, G.; Fortin, J.A. Early events of vesicular–arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 1988, 108, 211–218. [Google Scholar] [CrossRef]
- Hijri, M.; Sanders, I.R. The arbuscular mycorrhizal fungus Glomus intraradices is haploid and has a small genome size in the lower limit of eukaryotes. Fungal Genet. Biol. 2004, 41, 253–261. [Google Scholar] [CrossRef]
- Sánchez, S.; Gómez, E.; Martín, M.; De Miguel, A.M.; Urban, A.; Barriuso, J. Experiments on the life cycle and factors affecting reproduction of Sphaerosporella brunnea provide evidence for rapid asexual propagation by conidiospores and for homothallism in an ectomycorrhizal competitor of cultivated truffle species. Fungal Ecol. 2014, 8, 59–65. [Google Scholar] [CrossRef]
- Arnot, J.A.; Gobas, F.A.P.C. A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 2006, 14, 257–297. [Google Scholar] [CrossRef]
- Guidi, W.; Kadri, H.; Labrecque, M. Establishment techniques to using willow for phytoremediation on a former oil refinery in southern Quebec: Achievements and constraints. Chem. Ecol. 2012, 28, 49–64. [Google Scholar] [CrossRef]
- Courchesne, F.; Turmel, M.-C.; Cloutier-Hurteau, B.; Constantineau, S.; Munro, L.; Labrecque, M. Phytoextraction of soil trace elements by willow during a phytoremediation trial in Southern Québec, Canada. Int. J. Phytoremediat. 2017, 19, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal plants: Their ecology and relevance. New Phytol. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, K.; Huang, J.; Nara, K.; Chen, Y.; Shen, Z.; Lian, C. Inoculation of ectomycorrhizal fungi contributes to the survival of tree seedlings in a copper mine tailing. J. For. Res. 2015, 20, 493–500. [Google Scholar] [CrossRef]
- Ma, Y.; He, J.; Ma, C.; Luo, J.; Li, H.; Liu, T.; Polle, A.; Peng, C.; Luo, Z.-B. Ectomycorrhizas with Paxillus involutus enhance cadmium uptake and tolerance in Populus × canescens. Plant Cell Environ. 2014, 37, 627–642. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Sun, H.; Chen, Y.; Pan, H.; Yang, X.; Rafiq, T. Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS ONE 2014, 9, e108568. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Meers, E.; Vervaeke, P.; De Vos, B.; Quataert, P.; Tack, F.M. Growth and trace metal accumulation of two Salix clones on sediment-derived soils with increasing contamination levels. Chemosphere 2005, 58, 995–1002. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, D.; Brereton, N.J.; Marchand, L.; Brisson, J.; Pitre, F.E.; Labrecque, M. Complementarity of three distinctive phytoremediation crops for multiple-trace element contaminated soil. Sci. Total Environ. 2018, 610, 1428–1438. [Google Scholar] [CrossRef]
- Violante, A.; Cozzolino, V.; Perelomov, L.; Caporale, A.G.; Pigna, M. Mobility and bioavailability of heavy metals and metalloids in soil environments. J. Soil Sci. Plant Nutr. 2010, 10, 268–292. [Google Scholar] [CrossRef] [Green Version]
- Alloway, B.J. Bioavailability of Elements in Soil. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 351–373. [Google Scholar] [CrossRef]
- Chaudhry, F.; Wallace, A.; Mueller, R. Barium toxicity in plants. Commun. Soil Sci. Plant Anal. 1977, 8, 795–797. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Sell, J.; Kayser, A.; Schulin, R.; Brunner, I. Contribution of Ectomycorrhizal Fungi to Cadmium Uptake of Poplars and Willows from a Heavily Polluted Soil. Plant Soil 2005, 277, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Liang, Y.; Han, X.; Chiu, T.-Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sampling Plot | ||||||
|---|---|---|---|---|---|---|
| Metal | Unit | 1 | 2 | 3 | 4 | 5 |
| Silver (Ag) | mg/kg | <0.5 | 8.5 | 3.1 | 0.6 | 2.3 |
| Arsenic (As) | mg/kg | 8 | 17 | 27 | 13 | 17 |
| Barium (Ba) | mg/kg | 190 | 630 | 530 | 830 | 540 |
| Cadmium (Cd) | mg/kg | 1.6 | 37 | 12 | 4 | 7.4 |
| Chrome (Cr) | mg/kg | 65 | 74 | 88 | 71 | 93 |
| Cobalt (Co) | mg/kg | 18 | 13 | 21 | 18 | 16 |
| Copper(Cu) | mg/kg | 110 | 550 | 680 | 420 | 600 |
| Tin (Sn) | mg/kg | 56 | 310 | 730 | 88 | 440 |
| Manganese (Mn) | mg/kg | 530 | 670 | 1200 | 520 | 790 |
| Molybdenum (Mo) | mg/kg | 2 | 8 | 12 | 3 | 7 |
| Nickel (Ni) | mg/kg | 61 | 65 | 150 | 88 | 99 |
| Lead (Pb) | mg/kg | 150 | 1100 | 2400 | 430 | 1800 |
| Zinc (Zn) | mg/kg | 380 | 4400 | 5400 | 2000 | 2300 |
| pH | 7.18 | 7.09 | 7.40 | 7.12 | 7.09 | |
| Barium | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 262.7 | 38.33 | 337.5 | 47.94 | 339.8 | 52.62 | 464.8 | 64.19 | 362.9 | 39.5 |
| 2019 | 247.9 | 35.27 | 298.1 | 38.43 | 247.5 | 39.8 | 264.3 * | 22.84 | 302.3 | 38.85 |
| Cadmium | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 5.64 | 1.03 | 7.6 | 1.91 | 7.16 | 1.28 | 6.99 | 1.02 | 6.35 | 1.23 |
| 2019 | 5.4 | 1.08 | 7.17 | 1.71 | 7.55 | 1.58 | 4.83 * | 0.72 | 4.89 | 0.75 |
| Copper | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 375.4 | 74.65 | 377.8 | 88.09 | 948.8 | 295.88 | 1261.6 | 476.76 | 526.7 | 94.17 |
| 2019 | 601.4 | 265.34 | 324.2 | 68.66 | 581.6 | 105.64 | 351.4 | 65.09 | 390.3 * | 61.04 |
| Lead | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 587.2 | 128.68 | 781.5 | 208.51 | 1010.4 | 178.19 | 1334 | 258.36 | 1672.7 | 931.39 |
| 2019 | 557 | 123.23 | 713.7 | 194.74 | 792 | 175.93 | 594.3 * | 156.94 | 622.7 | 124.49 |
| Nickel | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 87.1 | 9.29 | 107.2 | 12.74 | 114.1 | 13.45 | 118.2 | 12.89 | 93.2 | 11.67 |
| 2019 | 88.6 | 13.04 | 68.5 * | 6.99 | 95.9 | 16.47 | 79.5 * | 9.98 | 62.6 * | 4.42 |
| Tin | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 225.5 | 60.46 | 337.6 | 111.24 | 559.6 | 117.66 | 542.8 | 101.18 | 497.5 | 155.08 |
| 2019 | 244.6 | 62.51 | 247 | 72.06 | 410.7 | 123.79 | 265.9 * | 88.58 | 218.5 | 53.74 |
| Zinc | CN | SX | AM | EM | XX | |||||
| Year | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. | Mean | Std. Err. |
| 2016 | 1753.9 | 300.35 | 1697.7 | 324.05 | 1992.7 | 325.54 | 2063.6 | 249.48 | 2001.3 | 355.89 |
| 2019 | 2070.1 | 393.19 | 1346 * | 280.25 | 1656.3 | 259.44 | 1363.6 * | 236.71 | 1455.2 | 236.43 |
| Metal Decrease/Increase % and Plant Dry Biomass Tukey’s HSD Comparisons | |||||
|---|---|---|---|---|---|
| CN | SX | AM | EM | XX | |
| Ba | N.S. | N.S. | N.S. | N.S. | N.S. |
| Cd | N.S. | N.S. | N.S. | N.S. | N.S. |
| Cu | B | B | AB | A | AB |
| Pb | B | AB | AB | A | AB |
| Ni | N.S. | N.S. | N.S. | N.S. | N.S. |
| Sn | B | AB | AB | A | AB |
| Zn | N.S. | N.S. | N.S. | N.S. | N.S. |
| Biomass | B | B | A | B | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagher, D.J.; Pitre, F.E.; Hijri, M. Ectomycorrhizal Fungal Inoculation of Sphaerosporella brunnea Significantly Increased Stem Biomass of Salix miyabeana and Decreased Lead, Tin, and Zinc, Soil Concentrations during the Phytoremediation of an Industrial Landfill. J. Fungi 2020, 6, 87. https://doi.org/10.3390/jof6020087
Dagher DJ, Pitre FE, Hijri M. Ectomycorrhizal Fungal Inoculation of Sphaerosporella brunnea Significantly Increased Stem Biomass of Salix miyabeana and Decreased Lead, Tin, and Zinc, Soil Concentrations during the Phytoremediation of an Industrial Landfill. Journal of Fungi. 2020; 6(2):87. https://doi.org/10.3390/jof6020087
Chicago/Turabian StyleDagher, Dimitri J., Frédéric E. Pitre, and Mohamed Hijri. 2020. "Ectomycorrhizal Fungal Inoculation of Sphaerosporella brunnea Significantly Increased Stem Biomass of Salix miyabeana and Decreased Lead, Tin, and Zinc, Soil Concentrations during the Phytoremediation of an Industrial Landfill" Journal of Fungi 6, no. 2: 87. https://doi.org/10.3390/jof6020087
APA StyleDagher, D. J., Pitre, F. E., & Hijri, M. (2020). Ectomycorrhizal Fungal Inoculation of Sphaerosporella brunnea Significantly Increased Stem Biomass of Salix miyabeana and Decreased Lead, Tin, and Zinc, Soil Concentrations during the Phytoremediation of an Industrial Landfill. Journal of Fungi, 6(2), 87. https://doi.org/10.3390/jof6020087