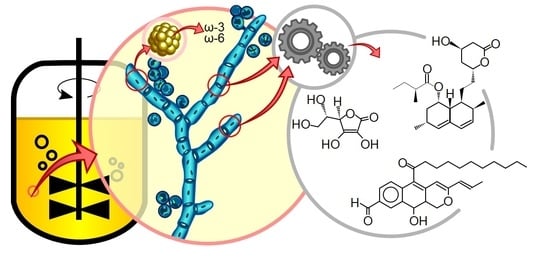
Use of the Versatility of Fungal Metabolism to Meet Modern Demands for Healthy Aging, Functional Foods, and Sustainability
Abstract
:1. Health and Modern Food Demands
2. Natural Food Additives from Fungi
3. Benefits, Research, and Industrial Applications of Fungal Metabolites
4. Mushrooms as Functional Foods for Preventing Aging-Related Non-Transmissible Chronic Diseases (NTCD)
5. Toward a Sustainable Production of Fungal Metabolites
6. Conclusions
Authors Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Piffieri, F.; Aujard, F. Caloric restriction, longevity and aging: Recent contributions from human and non-human primate studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109702. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Accardi, G.; Caruso, C.; Candore, G. Effects of nutraceuticals of Mediterranean diet on aging and longevity. In The Mediterranean Diet: An Evidence-Based Approach, 2nd ed.; Preedy, V.R., Wastson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 547–553. [Google Scholar] [CrossRef]
- Zhang, Z.; He, S.; Cao, X.; Ye, Y.; Yang, L.; Wang, J.; Liu, H.; Sun, H. Potential prebiotic activities of soybean peptides Maillard reaction products on modulating gut microbiota to alleviate aging-related disorders in D-galactose-induced ICR mice. J. Funct. Foods 2020, 65, 103729. [Google Scholar] [CrossRef]
- Cava, E.; Fontana, L. Will calorie restriction work in humans? Aging 2013, 5, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Chang, C.J.; Young, J.D. Antiaging effects of bioactive molecules isolated from plants and fungi. Med. Res. Rev. 2019, 39, 1515–1552. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current prospects of nutraceuticals: A review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Ruchi, S. Role of nutraceuticals in health care: A review. Int. J. Green Pharm. 2017, 11, S385–S394. [Google Scholar] [CrossRef]
- Padmavathi, D. A general review on “nutraceuticals”: Its golden health impact over human community. Int. J. Food. Sci. Nut. 2018, 3, 214–217. [Google Scholar]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Meyer, V.; Basenko, E.Y.; Philipp Benz, J.; Braus, G.H.; Caddick, M.X.; Csukai, M.; Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Kumari, K. Mushrooms as source of dietary fiber and its medicinal value: A review article. J. Pharmacogn. Phytochem. 2020, 9, 2075–2078. [Google Scholar]
- Kato, T.; Azegami, J.; Yokomori, A.; Dohra, H.; Enshasy, H.A.; Park, E.Y. Genomic analysis of a riboflavin—Overproducing Ashbya gossypii mutant isolated by disparity mutagenesis. BMC Genom. 2020, 21, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesus, L.F.M.C. Produção de β-Galactosidase Por Fungos Filamentosos: Screening, Purificação e Caracterização Bioquímica. Master’s Thesis, Universidade Estadual Paulista “Julio Mesquita Filho”, UNESP, São Paulo, Brazil, 2020. [Google Scholar]
- Liu, D.; Chen, Y.Q.; Xiao, X.W.; Zhong, R.T.; Yang, C.F.; Liu, B.; Zhao, C. Nutrient properties and nuclear magnetic resonance-based metabonomic analysis of macrofungi. Foods 2019, 8, 397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive compounds and antioxidant activity exhibit high intraspecific variability in Pleurotusostreatus mushrooms and correlate well with cultivation performance parameters. World J. Microb. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, N.; Fujiwara, S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids 2020, 52, 181–197. [Google Scholar] [CrossRef]
- Chan, L.G.; Dias, F.F.; Saarni, A.; Cohen, J.; Block, D.; Taha, A.Y.; de Moura Bell, J.M. Scaling up the bioconversion of cheese whey permeate into fungal oil by Mucor circinelloides. J. Am. Oil Chem. Soc. 2020, 97, 703–716. [Google Scholar] [CrossRef]
- Mamani, L.D.G.; Magalhães, A.I., Jr.; Ruan, Z.; de Carvalho, J.C.; Soccol, C.R. Industrial production, patent landscape, and market trends of arachidonic acid-rich oil of Mortierella alpina. Biotech. Res. Innov. 2019, 3, 103–119. [Google Scholar] [CrossRef]
- Anand, S.; Singh, K.S.; Aggarwal, D. Expanding Avenues for Probiotic Yeast. In Microbial Cell Factories, 1st ed.; Sharma, D., Saharan, B.S., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 125–141. [Google Scholar]
- Baggio, L.M.; Panagio, L.A.; Gasparin, F.G.M.; Sartori, D.; Celligoi, M.A.P.C.; Baldo, C. Production of fibrinogenolytic and fibrinolytic enzymes by a strain of Penicillium sp. isolated from contaminated soil with industrial effluent. Acta Sci. Health Sci. 2019, 41, e40606. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Zhang, N.; Sun, Z.; Shi, Y.; Cao, X.; Wang, H. Purification and characterization of a fibrinolytic enzyme from Rhizopus microsporus var. tuberosus. Food Technol. Biotech. 2015, 53, 243–248. [Google Scholar] [CrossRef]
- Huang, Z.; Brennan, C.S.; Zheng, H.; Mohan, M.S.; Stipkovits, L.; Liu, W.; Kulasiri, D.; Guan, W.; Zhao, H.; Liu, J. The effects of fungal lipase-treated milk lipids on bread making. LWT 2020, 109455. [Google Scholar] [CrossRef]
- Hjortmo, S.B.; Hellström, A.M.; Andlid, T.A. Production of folates by yeasts in Tanzanian fermented togwa. FEMS Yeast Res. 2008, 8, 781–787. [Google Scholar] [CrossRef] [Green Version]
- Copetti, M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019, 25, 52–56. [Google Scholar] [CrossRef]
- Dikshit, R.; Tallapragada, P. Comparative Study of Natural and Artificial Flavoring Agents and Dyes. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: London, UK, 2018; Volume 7, pp. 83–111. [Google Scholar]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Devi, P.R.; Ravi, A.V. Fungal Pigments: Potential coloring compounds for wide ranging applications in textile dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef]
- [ACS] American Chemical Society. Dyes, Pigments and Inks. 2020. Available online: https://www.acs.org/content/acs/en/careers/college-to-career/chemistry-careers/dyes-pigments-ink.html (accessed on 8 September 2020).
- [FDA] U.S. Food and Drug Administration. Color Additives History. 2017. Available online: https://www.fda.gov/industry/color-additives/color-additives-history#:~:text=A%20color%20additive%2C%20as%20defined,or%20to%20the%20human%20body.&text=One%20of%20the%20U.S.%20Food,are%20safely%20and%20appropriately%20used (accessed on 8 September 2020).
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Microbial production of food grade pigments. Food Technol. Biotech. 2006, 44, 313–321. [Google Scholar]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—Challenges and the way forward. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Lebeau, J.; Petit, T.; Clerc, P.; Dufossé, L.; Caro, Y. Isolation of two novel purple naphtoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium oxysporum. Biotech. Prog. 2019, 35. [Google Scholar] [CrossRef] [Green Version]
- Slack, G.J.; Puniani, E.; Frisvad, J.C.; Samson, R.A.; Miller, J.D. Secondary metabolites from Eurotium species, Aspergillus calidoustus and A. insuetus common in Canadian homes with a review of their chemistry and biological activities. Mycol. Res. 2009, 113, 480–490. [Google Scholar] [CrossRef]
- Mondal, S.; Pandit, S.G.; Puttananjaiah, M.H.; Harohally, N.V.; Dhale, M.A. Structural and functional characterization of new pigment molecule monashin from Monascuspurpureus CFR410-11. Process. Biochem. 2019, 82, 173–178. [Google Scholar] [CrossRef]
- Wu, H.C.; Cheng, M.J.; Wu, M.D.; Chen, J.J.; Chen, Y.L.; Chang, H.S.; Chen, K.P. Secondary metabolites from the fermented rice of the fungus Monascus purpureus and their bioactivities. Nat. Prod. Res. 2019, 33, 3541–3550. [Google Scholar] [CrossRef]
- Kraboun, K.; Kongbangkerd, T.; Rojsuntornkitti, K.; Phanumong, P. Factors and advances on fermentation of Monascus sp. for pigments and monacolin K production: A review. Int. Food. Res. J. 2019, 26, 751–761. [Google Scholar]
- Shi, Y.C.; Pan, T.M.; Liao, V.H.C. Monascin from Monascus-fermented products reduces oxidative stress and amyloid-beta toxicity via DAF-16/FOXO in Caenorhabditis elegans. J. Agric. Food. Chem. 2016, 64, 7114–7120. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Chen, T.H.; Lee, B.H.; Hsu, Y.W.; Pan, T.M. Monascin and ankaflavin act as natural AMPK activators with PPARα-agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 2014, 64, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, M.; Shum-Chéong-Sing, A.; Dufossé, L.; Fouillaud, M. Statistical optimization of the physico-chemical parameters for pigment production in submerged fermentation of Talaromyces albobiverticillius 30548. Microorganisms 2020, 8, 711. [Google Scholar] [CrossRef]
- Palacio-Barrera, A.M.; Areiza, D.; Zapata, P.; Atehortúa, L.; Correa, C.; Peñuela-Vásquez, M. Induction of pigment production through media composition, abiotic and biotic factors in two filamentous fungi. Biotechnol. Rep. 2018, 20, e00308. [Google Scholar] [CrossRef]
- Osemwegie, O.O.; Adetunji, C.O.; Ayeni, E.A.; Adejobi, O.I.; Arise, R.O.; Nwonuma, C.O.; Oghenekaro, A.O. Exopolysaccharides from bacteria and fungi: Current status and perspectives in Africa. Heliyon 2020, 6, e04205. [Google Scholar] [CrossRef]
- Mahapatra, S.; Banerjee, D. Fungal exopolysaccharide: Production, composition and applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Iyyappan, J.; Bharathiraja, B.; Baskar, G.; Kamalanaban, E. Process optimization and kinetic analysis of malic acid production from crude glycerol using Aspergillus niger. Bioresour. Technol. 2019, 281, 18–25. [Google Scholar] [CrossRef]
- Saeed, S.; Aslam, S.; Mehmood, T.; Naseer, R.; Nawaz, S.; Mujahid, H.; Firyal, S.; Anjum, A.A.; Sultan, A. Production of gallic acid under solid-state fermentation by utilizing waste from food processing industries. Waste Biomass Valoriz. 2020, 1–9. [Google Scholar] [CrossRef]
- Costa, C.R.L.M.; Menolli, R.A.; Osaku, E.F.; Tramontina, R.; Melo, R.H.; Amaral, A.E.; Duarte, P.A.D.; Carvalho, M.M.; Smiderle, F.R.; Silva, J.L.C.; et al. Exopolysaccharides from Aspergillus terreus: Production, chemical elucidation and immunoactivity. Int. J. Biol. Macromol. 2019, 139, 654–664. [Google Scholar] [CrossRef]
- Fooladi, T.; Soudi, M.R.; Alimadadi, N.; Savedoroudi, P.; Heravi, M.M. Bioactive exopolysaccharide from Neopestalotiopsis sp. strain SKE15: Production, characterization and optimization. Int. J. Biol. Macromol. 2019, 129, 127–139. [Google Scholar] [CrossRef]
- Xu, X.; Xu, R.; Jia, Q.; Feng, T.; Huang, Q.; Ho, C.T.; Song, S. Identification of dihydro-β-ionone as a key aroma compound in addition to C8 ketones and alcohols in Volvariella volvacea mushroom. Food Chem. 2019, 293, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zeng, H.; Meng, Y.; Xu, D.; Zhang, B.; Xin, B.; Liu, S.; Zhai, B.; Yu, F.; Zhu, M.; et al. Continuous natamycin production by using immobilized Streptomyces gilvosporeus Z8 via repeated batch culture. J. Chem. Technol. Biotechnol. 2020, 95, 73–77. [Google Scholar] [CrossRef]
- Bin, C.; Fangming, Y.; Zhi, J. Mogroside V-producing endophytic fungi isolated from Siraitia grosvenorii. Planta Med. 2020, 86, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Kallscheuer, N. Engineered microorganisms for the production of food additives approved by the European Union—A systematic analysis. Front. Microbiol. 2018, 9, 1746. [Google Scholar] [CrossRef]
- Kovilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2019, 95, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Lin, Z.; Huang, C.; Zhang, Y.; Wang, Z.; Tang, Y.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli using CRISPR-Cas9 mediated genome editing. Metab. Eng. 2015, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Tramontin, L.R.R.; Kildegaard, K.R.; Sudarsan, S.; Borodina, I. Enhancement of astaxanthin biosynthesis in oleaginous yeast Yarrowialipolytica via microalgal pathway. Microorganisms 2019, 7, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harith, Z.T.; Charalampopoulos, D.; Chatzifragkou, A. Rapeseed meal hydrolysate as substrate for microbial astaxanthin production. Biochem. Eng. J. 2019, 151, 107330. [Google Scholar] [CrossRef]
- Hwang, S.W.; Choi, H.I.; Sim, S.J. Acidic cultivation of Haematococcuspluvialis for improved astaxanthin production in the presence of a lethal fungus. Bioresour. Technol. 2019, 278, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ip, P.F.; Wong, K.H.; Chen, F. Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process. Biochem. 2004, 39, 1761–1766. [Google Scholar] [CrossRef]
- Tang, D.Y.Y.; Khoo, K.S.; Chew, K.W.; Tao, Y.; Ho, S.H.; Show, P.L. Potential utilization of bioproducts from microalgae for the quality enhancement of natural products. Bioresour. Technol. 2020, 304, 122997. [Google Scholar] [CrossRef] [PubMed]
- Villena, G.K.; Kitazono, A.A.; Hernández-Macedo, M.L. Bioengineering Fungi and Yeast for the Production of Enzymes, Metabolites, and Value-Added Compounds. In Fungal Biotechnology and Bioengineering; Hesham, A.E.L., Upadhyay, R.S., Sharma, G.D., Manoharachary, C., Gupta, V.K., Eds.; Springer: Cham, Switzerland, 2020; pp. 209–237. [Google Scholar] [CrossRef]
- Sun, X.; Su, X. Harnessing the knowledge of protein secretion for enhanced protein production in filamentous fungi. World J. Microbiol. Biotech. 2019, 35, 54. [Google Scholar] [CrossRef] [PubMed]
- Kumitch, H.M.; Stone, A.; Nosworthy, M.G.; Nickerson, M.T.; House, J.D.; Korber, D.R.; Tanaka, T. Effect of fermentation time on the nutritional properties of pea protein-enriched flour fermented by Aspergillus oryzae and Aspergillus niger. Cereal Chem. 2019, 97, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Gmoser, R.; Fristedt, R.; Larsson, K.; Undeland, I.; Taherzadeh, M.J.; Lennartsson, P.R. From stale bread and brewers spent grain to a new food source using edible filamentous fungi. Bioengineered 2020, 11, 582–598. [Google Scholar] [CrossRef]
- Asfour, H.Z.; Awan, Z.A.; Bagalagel, A.A.; Elfaky, M.A.; Abdelhameed, R.F.A.; Elhady, S.S. Large-scale production of bioactive terrein by Aspergillus terreus strain s020 isolated from the Saudi Coast of the Red Sea. Biomolecules 2019, 9, 480. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Lee, S.J.; Jung, J.E.; Kim, J.S.; Lee, N.H.; Yi, H.K. Terrein reduces age-related inflammation induced by oxidative stress through Nrf2/ERK1/2/HO-1 signalling in aged HDF cells. Cell Biochem. Funct. 2015, 33, 479–486. [Google Scholar] [CrossRef]
- [WHO]. WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications-detail-redirect/who-report-on-cancer-setting-priorities-investing-wisely-and-providing-care-for-all (accessed on 28 August 2020).
- Ren, F.; Zhu, S.; Wang, B.; Li, L.; Liu, X.; Su, R.; Che, Y. Hypocriols AF heterodimeric botryane ethers from Hypocrea sp., an insect-associated fungus. J. Nat. Prod. 2016, 79, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, S.S.; Yuan, W.Y.; Wei, J.J.; Zhao, Y.X.; Luo, D.Q. Carotane-type sesquiterpenes from cultures of the insect pathogenic fungus Isaria fumosorosea. J. Asian Nat. Prod. Res. 2017, 21, 1–7. [Google Scholar] [CrossRef]
- Chen, P.; Qin, H.J.; Li, Y.W.; Ma, G.X.; Yang, J.S.; Wang, Q. Study on chemical constituents of an edible mushroom Volvariella volvacea and their antitumor activity in vitro. Nat. Prod. Res. 2018, 34, 1–6. [Google Scholar] [CrossRef]
- Chowdhury, N.S.; Sohrab, M.H.; Rana, M.S.; Hasan, C.M.; Jamshidi, S.; Rahman, K.M. Cytotoxic naphthoquinone and azaanthraquinone derivatives from an endophytic Fusarium solani. J. Nat. Prod. 2017, 80, 1173–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ory, L.; Nazih, E.H.; Daoud, S.; Mocquard, J.; Bourjot, M.; Margueritte, L.; Delsuc, M.A.; Bard, J.M.; Pouchus, Y.F.; Bertrand, S.; et al. Targeting bioactive compounds in natural extracts—Development of a comprehensive workflow combining chemical and biological data. Anal. Chim. Acta 2019, 1070, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta. Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Abdel-Azeem, A.M.; Abdel-Azeem, M.A.; Darwish, A.G.; Nafady, N.A.; Ibrahim, N.A. Fusarium: Biodiversity, Ecological Significances, and Industrial Applications. In Recent Advancement in White Biotechnology through Fungi, Fungal Biology; Yadav, A.N., Singh, S., Mishra, S., Gupta, A., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 3, pp. 201–261. [Google Scholar]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Hameed, A.; Hussain, S.A.; Yang, J.; Ijaz, U.M.; Liu, Q.; Suleria, H.A.R.; Song, Y. Antioxidants potential of the filamentous fungi (Mucor circinelloides). Nutrients 2017, 9, 1101. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Pan, F.; El-Kashef, D.H.; Kalscheuer, R.; Müller, W.E.G.; Lee, J.; Feldbrügge, M.; Mándi, A.; Kurtán, T.; Liu, Z.; Wu, W.; et al. Cladosins L-O, new hybrid polyketides from the endophytic fungus Cladosporium sphaerospermum WBS017. Eur. J. Med. Chem. 2020, 191, 112159. [Google Scholar] [CrossRef]
- Al-Rabia, M.W.; Mohamed, G.A.; Ibrahim, S.R.M.; Asfoura, H.Z. Anti-inflammatory ergosterol derivatives from the endophytic fungus Fusarium chlamydosporum. Nat. Prod. Res. 2020. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, B.; Kang, Y.; Li, Y.; Cui, Y.; Liu, W.; Xiang, Z. A neuroinflammation inhibitor, hypoxylon xanthone A, from soil fungus Hypoxylon sp. Lett. Org. Chem. 2020, 17, 116–120. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, S.; Gu, G.; Xu, D.; Zhang, X.; Lai, D.; Zhou, L. Rhizovagine A, an unusual dibenzo-a-pyrone alkaloid from the endophytic fungus Rhizopycnisvagum nitaf 22. RSC Adv. 2020, 10, 27894–27898. [Google Scholar] [CrossRef]
- Chapla, V.M.; Honório, A.E.; Gubiani, J.R.; Vilela, A.F.L.; Young, M.C.M.; Cardoso, C.L.; Pavan, F.R.; Cicarelli, R.M.; Ferreira, P.M.P.; Bolzani, V.S.; et al. Acetylcholinesterase inhibition and antifungal activity of cyclohexanoids from the endophytic fungus Saccharicola sp. Phytochem. Lett. 2020, 39, 116–123. [Google Scholar] [CrossRef]
- Qi, J.; Zhao, P.; Zhao, L.; Jia, A.; Liu, C.; Zhang, L.; Xia, X. Anthraquinone derivatives from a sea cucumber-derived Trichoderma sp. fungus with antibacterial activities. Chem. Nat. Compd. 2020, 56, 112–114. [Google Scholar] [CrossRef]
- Adpressa, D.A.; Connolly, L.R.; Konkel, Z.M.; Neuhaus, G.F.; Chang, X.L.; Pierce, B.R.; Smith, K.M.; Freitag, M.; Loesgen, S. A metabolomics-guided approach to discover Fusarium graminearum metabolites after removal of a repressive histone modification. Fungal Genet. Biol. 2019, 132, 103256. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.H.; Chiou, S.H.; Huang, C.H.; Yang, W.B.; Wong, C.H. The lifespan-promoting effect of acetic acid and Rishi polysaccharide. Bioorg. Med. Chem. 2009, 17, 7831–7840. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Rajtilak, M.; Matt, L.; Brian, M.; Rakesh, M.; Subhash, M.; Carol, C.W.; Christine, S.; Kanniah, R.; Jeffrey, C. The Aspergillus flavus spermidine synthase (spds) gene, is required for normal development, aflatoxin production, and pathogenesis during infection of Maize Kernels. Front. Plant Sci. 2018, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kała, K.; Kryczyk-Poprawa, A.; Rzewińska, A.; Muszyńska, B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur. Food. Res. Technol. 2020, 246, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Jing, T.; Meng, Q.; Liu, C.; Hu, S.; Ma, Y.; Liu, Y.; Lu, J.; Cheng, Y.; Wang, D.; et al. Studies on the antidiabetic activities of Cordyceps militaris extract in diet-streptozotocin-induced diabetic Sprague-Dawley rats. Biomed Res. Int. 2014, 2014, 160980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Sun, H.; Hao, Y.; Zheng, X.; Song, Q.; Dai, S.; Zhu, Z. Chemical structure and inhibition on α-glucosidase of the polysaccharides from Cordyceps militaris with different developmental stages. Int. J. Biol. Macromol. 2020, 148, 722–736. [Google Scholar] [CrossRef]
- He, S.; Wang, Y.; Xie, J.; Gao, H.; Li, X.; Huang, Z. 1H NMR-based metabolomic study of the effects of flavonoids on citrinin production by Monascus. Food Res. Int. 2020, 137, 109532. [Google Scholar] [CrossRef]
- Guo, L.; Li, K.; Kang, J.S.; Kang, N.J.; Son, B.G.; Choi, Y.W. Strawberry fermentation with Cordyceps militaris has anti-adipogenesis activity. Food Biosci. 2020, 35, 100576. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Ashraf, M.S.; Adnan, M.; Hadi, S. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic Cordyceps medicinal fungus and its nutraceutical and therapeutic potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef]
- Guo, P.; Kai, Q.; Gao, J.; Lian, Z.Q.; Wu, C.M.; Wu, C.A.; Zhu, H.B. Cordycepin prevents hyperlipidemia in hamsters fed a high-fat diet via activation of AMP-activated protein kinase. J. Pharmacol. Sci. 2010, 113, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Kopalli, S.R.; Cha, K.M.; Lee, S.H.; Hwang, S.Y.; Lee, Y.J.; Koppula, S.; Kim, S.K. Cordycepin, an active constituent of nutrient powerhouse and potential medicinal mushroom Cordyceps militaris Linn., ameliorates age-related testicular dysfunction in rats. Nutrients 2019, 11, 906. [Google Scholar] [CrossRef] [Green Version]
- Kerrigan, R.W. Agaricus subrufescens, a cultivated edible and medicinal mushroom, and its synonyms. Mycologia 2005, 97, 12–24. [Google Scholar] [CrossRef]
- Taofiq, O.; Rodrigues, F.; Barros, L.; Peralta, R.M.; Barreiro, M.F.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Agaricus blazei Murrill from Brazil: An ingredient for nutraceutical and cosmeceutical applications. Food Funct. 2019, 10, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navegantes-Lima, K.C.; Monteiro, V.V.S.; Gaspar, S.L.F.; Oliveira, A.L.B.; de Oliveira, J.P.; Reis, J.F.; Gomes, R.S.; Rodrigues, C.A.; Stutz, H.; Sovrani, V.; et al. Agaricus brasiliensis mushroom protects against sepsis by alleviating oxidative and inflammatory response. Front. Immunol. 2020, 11, 1238. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Chen, C.Y.; Hsiao, W.W.; Li, J.; Lin, Z.X.; Chu, F.H.; Yen, G.C.; Wang, S.Y. A mechanistic and empirical review of antcins, a new class of phytosterols of formosan fungi origin. J. Food Drug Anal. 2020, 28, 38–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Sugawara, Y.; Chen, S.; Beelman, R.B.; Tsuduki, T.; Tomata, Y.; Matsuyama, S.; Tsuji, I. Mushroom consumption and incident risk of prostate cancer in Japan: A pooled analysis of the Miyagi Cohort Study and the Ohsaki Cohort Study. Int. J. Cancer 2020, 146, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Torres-Mendoza, D.; Ortega, H.E.; Cubilla-Rios, L. Patents on endophytic fungi related to secondary metabolites and biotransformation applications. J. Fungi 2020, 6, 58. [Google Scholar] [CrossRef]
- Da Rocha, W.R.V.; Costa-Silva, T.A.; Agamez-Montalvo, G.S.; Feitosa, V.A.; Machado, S.E.F.; de Souza Lima, G.M.; Pessoa, A.J.; Alves, H.S. Screening and optimizing fermentation production of l-asparaginase by Aspergillus terreus strain S-18 isolated from the Brazilian Caatinga Biome. J. Appl. Microbiol. 2019, 126, 1426–1437. [Google Scholar] [CrossRef]
- Clarance, P.; Khusro, A.; Lalitha, J.; Sales, J.; Paul, A. Optimization of camptothecin production and biomass yield from endophytic fungus Fusarium solani strain ATLOY-8. J. Appl. Pharm. Sci. 2019, 9, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Mefteh, F.B.; Frikha, F.; Daoud, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Al-Anzi, B.S.; Oszako, T.; Gharsallah, N.; Belbahri, L. Response surface methodology optimization of an acidic protease produced by Penicillium bilaiae isolate TDPEF30, a newly recovered endophytic fungus from healthy roots of date palm trees (Phoenix dactylifera L.). Microorganisms 2019, 7, 74. [Google Scholar] [CrossRef] [Green Version]
- Atlı, B.; Yamaç, M.; Yıldız, Z.; Şőlener, M. Solid state fermentation optimization of Pleurotus ostreatus for lovastatin production. Pharm. Chem. J. 2019, 53, 858–864. [Google Scholar] [CrossRef]
- Ravuri, M.; Shivakumar, S. Optimization of conditions for production of lovastatin, a cholesterol lowering agent, from a novel endophytic producer Meyerozyma guilliermondii. J. Biol. Product. Nat. 2020, 10, 192–203. [Google Scholar] [CrossRef]
- Khan, Y.M.; Munir, H.; Anwar, Z. Optimization of process variables for enhanced production of urease by indigenous Aspergillus niger strains through response surface methodology. Biocatal. Agric. Biotechnol. 2019, 20, 101202. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, E.S.R.; Zaki, A.G.; Ahmed, A.S.; Ismaiel, A.A. Production of the anticancer drug taxol by the endophytic fungus Epicoccum nigrum TXB502: Enhanced production by gamma irradiation mutagenesis and immobilization technique. Appl. Microbiol. Biotechnol. 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zaki, A.G.; El-Shatoury, E.H.; Ahmed, A.S.; Al-Hagar, O.E. Production and enhancement of the acetylcholinesterase inhibitor, huperzine A, from an endophytic Alternaria brassicae AGF041. Appl. Microbiol. Biotechnol. 2019, 103, 5867–5878. [Google Scholar] [CrossRef]
- Biswas, D.; Nazir, R.; Biswas, P.; Kumar, V.; Nandy, S.; Mukherjee, A.; Mukherjee, A.; Dey, A.; Pandey, D.K. Endophytic sources of diosgenin, a natural steroid with multiple therapeutic values. S. Afr. J. Bot. 2020. [Google Scholar] [CrossRef]
- Bouhri, Y.; Askun, T.; Tunca, B.; Deniz, G.; Aksoy, S.A.; Mutlu, M. The orange-red pigment from Penicillium mallochii: Pigment production, optimization, and pigment efficacy against Glioblastoma cell lines. Biocatal. Agric. Biotechnol. 2020, 23, 101451. [Google Scholar] [CrossRef]
- Xu, S.; Ren, N.; Liu, J.; Wu, Y.; Yuan, G. Improvement of vincamine production of endophytic fungus through inactivated protoplast fusion. Int. Microbiol. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, N.; Kato, S.; Kato, S.; Hidese, R.; Wagu, Y.; Sakoda, H.; Fujiwara, S. Agmatine production by Aspergillus oryzae is elevated by low pH during solid-state cultivation. Appl. Environ. Microbiol. 2018, 84, e00722-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barua, S.; Kim, J.Y.; Kim, J.Y.; Kim, J.H.; Lee, J.E. Therapeutic effect of agmatine on neurological disease: Focus on ion channels and receptors. Neurochem. Res. 2019, 44, 735–750. [Google Scholar] [CrossRef]
- [ADI] Alzheimer’s Disease International. World Alzheimer Report. In Attitudes to Dementia; Alzheimer’s Disease International: London, UK, 2019; Available online: https://www.alz.co.uk/research/world-report-2019 (accessed on 29 August 2020).
- Patel, R. A moldy application of MALDI: MALDI-ToF mass spectrometry for fungal identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteki, M.; Shahsavari, Z.; Simal-Gandara, J. Gas Chromatographic fingerprinting coupled to chemometrics for food authentication. Food Rev. Int. 2019, 36. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Y.; Zhang, X.; Xu, J.; Wang, H.; Zhao, L.; Wang, Y. Screening immunoactive compounds of Ganoderma lucidum spores by mass spectrometry molecular networking combined with in vivo Zebrafish assays. Front. Pharmacol. 2020, 11, 287. [Google Scholar] [CrossRef] [PubMed]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotech. 2017, 43, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Tian, Y.; Jiang, P.; Lin, Y.; Liu, X.; Yang, H. Recent advances in the application of metabolomics for food safety control and food quality analyses. Crit. Rev. Food Sci. Nutr. 2020, 1–22. [Google Scholar] [CrossRef]
- Hammerl, R.; Frank, O.; Schmittnägel, T.; Ehrmann, M.A.; Hofmann, T. Functional metabolome analysis of Penicillium roqueforti by means of differential off-line LC−NMR. J. Agric. Food Chem. 2019, 67, 5135–5146. [Google Scholar] [CrossRef]
- Alanne, A.L.; Issakainen, J.; Pihlaja, K.; Jokioja, J.; Sinkkonen, J. Metabolomic discrimination of the edible mushrooms Kuehneromyce smutabilis and Hypholom acapnoides (Strophariaceae, Agaricales) by NMR spectroscopy. Z. Naturforsch. 2019, 74, 201–210. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Fernández-Recamales, A. Metabolomics: An emerging tool for wine characterization and the investigation of health benefits. In Engineering Tools in the Beverage Industry: The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Wood Head Publishing: Sawston, UK, 2019; Volume 3, pp. 315–350. [Google Scholar] [CrossRef]
- Florentino-Ramos, E.; Villa-Ruano, N.; Hidalgo-Martínez, D.; Ramírez-Meraz, M.; Méndez-Aguilar, R.; Velásquez-Valle, R.; Zepeda-Vallejo, L.G.; Pérez-Hernández, N.; Becerra-Martínez, E. 1H NMR-based fingerprinting of eleven Mexican Capsicum annuum cultivars. Food Res. Int. 2019, 121, 12–19. [Google Scholar] [CrossRef]
- Gąsecka, M.; Siwulski, M.; Mleczek, M. Evaluation of bioactive compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J. Food Process. Preserv. 2017, e13386. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RDA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2020, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, L.P.; Vargas, E.A.; Madureira, F.D.; Faria, A.F.; Augusti, R. Development and validation of a novel analytical method to quantify aflatoxins in baby food samples by employing dispersive solid phase extraction with multi-walled carbon nanotubes. Food Anal. Methods 2020, 13, 1530–1537. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol, F.S.; Skalicka-Wozniak, K.; Georgiev, M.; Sener, B. Adulteration and safety issues in nutraceuticals and dietary supplements: Innocent or risky? In Nutraceuticals: Nanotechnology in the Agri-Food Industry; Grumezescu, A.M., Ed.; Academic Press: Cambridge, UK, 2016; Volume 4, pp. 153–182. [Google Scholar]
- Colosimo, R.; Gabriele, M.; Cifelli, M.; Longo, V.; Domenici, V.; Pucci, L. The effect of sourdough fermentation on Triticum dicoccum from Garfagnana: 1H NMR characterization and analysis of the antioxidant activity. Food Chem. 2020, 305, 125510. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, E.; Mantzouridou, F.T. Citric acid production from the integration of Spanish-style green olive processing wastewaters with white grape pomace by Aspergillus niger. Bioresour. Technol. 2019, 280, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [Green Version]
- Roukas, T.; Kotzekidou, P. Pomegranate peel waste: A new substrate for citric acid production by Aspergillus niger in solid-state fermentation under non-aseptic conditions. Environ. Sci. Pollut. Res. 2020, 27, 13105–13113. [Google Scholar] [CrossRef]
- Souza Filho, P.F.; Zamani, A.; Taherzadeh, M.J. Edible protein production by filamentous fungi using starch plant wastewater. Waste Biomass Valoriz. 2019, 10, 2487–2496. [Google Scholar] [CrossRef] [Green Version]
- Dulf, F.V.; Vodnar, D.C.; Toşa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef] [PubMed]





| Category | Active Component | Bioactivity | Fungal Source | References |
|---|---|---|---|---|
| Nutrient | Vitamin C (55) | Antioxidant | Dictyophora indusiata | [14] |
| Secondary Metabolites | Resveratrol | Antioxidant | Pleurotusostreatus | [15] |
| Agmatine (28) | Neurological benefits | Aspergillus oryzae | [16] | |
| ω-6 Polyunsaturated fatty acid | γ-linolenic acid | Anti-inflammatory | Mucor circinelloides | [17] |
| Arachidonic acid | Development of the nervous central system and enhancement of immune response | Mortierella alpina | [18] | |
| Probiotic | Whole cell | Increase of beneficial bacteria population in gastrointestinal tract | Saccharomyces boulardii | [19] |
| Nutraceutical Enzymes | Fibrino(geno)lytic enzymes | Antithrombotic | Penicillium sp. | [20] |
| Rhizopus microsporus | [21] | |||
| Lipase (Lipopan F) | Decrease glycemic response | Rhizopus oryzae | [22] | |
| Fortified Nutraceuticals | Folate in fermented maize-based porridge | Saccharomyces cerevisiae | [23] |
| Function | Compound Name and Structure | Fungus | Main Uses | References |
|---|---|---|---|---|
| Acidulant | Malic acid | A. niger | Candies, soft drinks, baked goods | [44] |
| Antioxidant | Gallic acid | A. niger | Any food susceptible to oxidation | [45] |
| Thickener agent | Galactomannans and β-1,3-glucans | Aspergillus terreus | Sauces, soups, puddings, fillings | [46] |
| Emulsifier | Sorbitol | Neopestalotiopsis sp. | Baked goods and desserts | [47] |
| Flavoring agent | Dihydro-β-ionone | Volvariellavolvacea | Beverages, ice creams, candies | [48] |
| Preservative | Natamycin | Streptomyces gilvosporeus | Fungicide used as biopreservative in dairy and meat products | [49] |
| Sweetener | Mogroside V | Diaporthe angelicae and Fusarium solani | Juices, soft drinks, cereals, confectionary, baked goods | [50] |
| Metabolite | Fungal Origin | Yield | Cytotoxic Activity | Reference | |||
|---|---|---|---|---|---|---|---|
| Human Tumor Cell | IC50 | Control | IC50 | ||||
| 7-Desmethyl-6-methylbostrycoidin (32) | F. solani | 7 mg/4.3 g extract | Breast (MDA MB 231) | 0.73 µM | Doxorubicin | 0.07 µM | [69] |
| Pancreatic (MIA PaCa2) | 0.64 µM | 0.04 µM | |||||
| Cervical (HeLa) | 0.71 µM | 0.05 µM | |||||
| Non small-cell lung (NCI HI975) | 0.34 µM | 0.03 µM | |||||
| Lung fibroblast (WI38) | 6.42 µM | 0.35 µM | |||||
| 7-Desmethylscorpinone (33) | F. solani | 5 mg/4.3 g extract | Breast (MDA MB 231) | 1.51 µM | Doxorubicin | 0.07 µM | [69] |
| Pancreatic (MIA PaCa2) | 0.98 µM | 0.04 µM | |||||
| Cervical (HeLa) | 0.96 µM | 0.05 µM | |||||
| Non small-cell lung (NCI HI975) | 0.61 µM | 0.03 µM | |||||
| Lung fibroblast (WI38) | 5.84 µM | 0.35 µM | |||||
| Ergosterol (43) | Penicillium chrysogenum | ni | Breast (MCF-7) | 0.10 mM | ni | ni | [70] |
| V.volvacea | 500 mg/725 g extract | Prostate (PC-3M) | 27.98 ± 0.97 µM | 5-Fluorouracil | 64.35 µM | [68] | |
| Ergosterol peroxide (44) | V. volvacea | 15 mg/725 g extract | Prostate (PC-3M) | 23.15 ± 1.54 µM | 5-Fluorouracil | 64.35 µM | [68] |
| 3β, 5α, 9α, 14 α-Tetrahydroxy-ergosta-7,22-dien-6-one (45) | V. volvacea | 5.9 mg/725 g extract | Liver (HepG2) | 20.72 ± 0.76 µM | 5-Fluorouracil | 54.74 µM | [68] |
| 3β, 5α, 9α-Trihydroxy-ergosta-7,22-dien-6-one (46) | V. volvacea | 12.5 mg/725 g extract | Gastric (SGC-7901) | 12.03 ± 0.77 µM | 5-Fluorouracil | 75.05 µM | [68] |
| Liver (HepG2) | 5.90 ± 0.44 µM | 54.74 µM | |||||
| Hypocriol A (24) | Hypocrea sp. | 50.3 mg/63.2 g extract | Colorectal (HCT116) | 18.6 ± 0.7 µM | Cisplatin | 18.8 ± 1.9 µM | [66] |
| Cervical (HeLa) | 7.7 ± 0.4 µM | 14.7 ± 0.8 µM | |||||
| Lung (A549) | 25.3 ± 2.5 µM | 13.8 ± 1.2 µM | |||||
| Breast (MCF-7) | 19.7 ± 0.4 µM | 17.6 ± 2.4 µM | |||||
| Hypocriol F (25) | Hypocrea sp. | 11.2 mg/63.2 g extract | Colorectal (HCT116) | 2.7 ± 0.6 µM | Cisplatin | 18.8 ± 1.9 µM | [66] |
| Cervical (HeLa) | 4.6 ± 0.1 µM | 14.7 ± 0.8 µM | |||||
| Lung (A549) | 15.3 ± 1.6 µM | 13.8 ± 1.2 µM | |||||
| Breast (MCF-7) | 23.6 ± 1.3 µM | 17.6 ± 2.4 µM | |||||
| Terrein (23) | A. terreus | 537.26 ± 23.42 g/kg extract | Colorectal (HCT-116) | 12.13 µM | Doxorubicin | 0.11 µM | [63] |
| Hepatocellular (HepG2) | 22.53 µM | 0.85 µM | |||||
| Trichocarane E (26) | I. fumosorosea | 30 mg/200 g extract | Breast (MDA) | 0.13 µg/mL | Cisplatin | 2.90 µg/mL | [67] |
| Breast (MCF-7) | 2.46 µg/mL | 1.14 µg/mL | |||||
| Ovary (SKOV-3) | 1.01 µg/mL | 3.80 µg/mL | |||||
| Cervical (Hela) | 2.32 µg/mL | 2.24 µg/mL | |||||
| Lung (A549) | 1.40 µg/mL | 2.13 µg/mL | |||||
| Liver (HepG2) | 1.87 µg/mL | 0.62 µg/mL | |||||
| Trichocarane F (27) | I. fumosorosea | 41 mg/200 g extract | Breast (MDA) | 0.89 µg/mL | Cisplatin | 2.90 µg/mL | [67] |
| Breast (MCF-7) | 4.38 µg/mL | 1.14 µg/mL | |||||
| Ovary (SKOV-3) | 1.46 µg/mL | 3.80 µg/mL | |||||
| Cervical (Hela) | 4.57 µg/mL | 2.24 µg/mL | |||||
| Lung (A549) | 1.66 µg/mL | 2.13 µg/mL | |||||
| Liver (HepG2) | 3.66 µg/mL | 0.62 µg/mL | |||||
| Fungal Species | Bioactive Compound | Bioactivity | Health Benefit | References | ||
|---|---|---|---|---|---|---|
| Target of Inhibitory Activity | Value | Control | ||||
| Cladosporium sphaerospermum | Cladosin L | Staphylococcus aureus | MIC = 25–50 µM | ni | Antibacterial | [78] |
| Fusarium chlamydosporum | Chlamydosterol A | 5-lipoxygenase (5-LOX) | IC50 = 3.06 µM | Indomethacin IC50 =1.13 µM | Anti-inflammatory | [79] |
| Hypoxylon sp. | Hypoxylon xanthone A | LPS-induced NO production | >70% at 1 µM | Minocycline >60% at 1 µM | Anti-neuroinflammatory | [80] |
| Rhizopycnisvagum | Rhizovagine A | Acetylcholinesterase enzyme | IC50 = 43.1 µM | Tacrine hydrochloride IC50 = 6.1 µM | Treatment for Alzheimer’s disease | [81] |
| Saccharicola sp. | Speciosin U | Acetylcholinesterase (huAChE-ICER) | IC50 = 0.037 ± 0.01 mg.mL−1 | Galantamine IC50 = 0.076 ± 0.01 mg.mL−1 | Treatment for Alzheimer’s disease | [82] |
| Trichoderma sp. | Coniothyrinone A | Vibrio anguillarum | MIC = 1.56 µM | Ciprofloxacin MIC = 0.625 µM | Antibacterial | [83] |
| Mushroom Species | Popular Name | Origin | Related Compounds | Health Benefits | References |
|---|---|---|---|---|---|
| G. lucidum | Reishi | China and Eastern Asia | Polysaccharides | Antioxidant activity related to DAF-16/FOXO activation | [85] |
| H.sinensis | Caterpillar | Tibet | Polysaccharides | Prebiotic properties related to insulin resistance and diabetes control | [87,88] |
| L. edodes | Shitake | Eastern Asia | Spermidine (39) | Reduction on age-dependent memory impairment | [91] |
| A. bisporus | Champignon | Eastern Europe | Lovastatin (40) | LDL-cholesterol lowering | [94] |
| C. cibarius | Chanterelle | [92] | |||
| I. badia | Bay bolete | [92] | |||
| L. edodes | Shitake | [92] | |||
| C. militaris | Caterpillar | China, Tibet | Polysaccharides | Immunomodulation improvement | [94,95] |
| Cordycepin (41) | Total and LDL-cholesterol lowering, reduction of hyperlipidemia caused by high-fat diets | [96] | |||
| A. subrufescens | Sun Mushroom | Eastern North America | Polyphenols, polysaccharides | Decrease of oxidative stress, preventing diseases like cancer and inflammation | [100,101,102] |
| A. cinnamomea | Niu-Chang-Chih | Taiwan | Antcins (42) | [103] |
| Fungal Species | Host Plant | Target Compound | Health Benefit | Methodology | Target Parameters | Reference |
|---|---|---|---|---|---|---|
| A.terreus | Coconut tree | L-Asparaginase | Treatment of acute lymphocytic leukemia | Factorial experimental design. Increase of scale(5-l bioreactor system). | pH, temperature, inoculum concentration. | [106] |
| F. solani | Chonemorpha fragrans | Camptothecin | Anticancer | Box–Behnken design using one factor at a time method. | Carbon and nitrogen sources, ethanol concentration, pH, temperature, stirring speed, incubation period, precursors and elicitors. | [107] |
| Penicillium bilaiae | Phoenix dactylifera | Acidic protease | Increasing in food digestibility | Response surface methodology. Plackett-Burman design. Box-Behnken design. | Temperature, initial pH, carbon and nitrogen sources, metal ions, detergents and enzyme inhibitors. | [108] |
| P. ostreatus | ni | Lovastatin (40) | Anti-hypercholesterolemic | Response surface methodology. | Nutrients, particle size of the solid substrate, temperature, incubation time. | [109] |
| Meyerozymaguilliermondii | leaves of Hibiscus rosa-sinensis | One parameter at time approach. | Nutrients, pH, inoculum size, temperature, addition of metallic ions, modulators, precursors. | [110] | ||
| A.niger | ni | Urease | Diuretic | Response surface methodology. | Strains, incubation time, temperature, pH, biomass, inoculum size, nitrogen content and moisture. | [111] |
| Spissiomycesendophytica | Balanophorafungosa | Melanin | Radioprotective, thermoregulator, antitumor, and antiviral | One parameter at time approach. | Inhibitors, culture medium, temperature, pH. | [112] |
| E. nigrum | Taxus baccata | Taxol | Anticancer | One parameter at a time approach. Mutant strains. | Culture medium, stirring speed, temperature, incubation period, pH, medium volume, inoculum age, inoculum size, carbon source, nitrogen source, phosphorus source, gamma radiation dose. | [113] |
| Alternaria brassicae | Huperzia serrata | Huperzine A | Acetylcholinesterase inhibitor | Multifactorial statistical approaches. Plackett–Burman. Central composite designs. | Culture medium composition, medium volume, inoculum age, inoculum size, incubation period, ethanol addition, pH, temperature. | [114] |
| F. oxysporum | Dioscoreazingiberensis | Diosgenin | Anti-cancer, anti-thrombic, anti-diabetic, cardioprotective, osteoarthritis protective activity | One parameter at time approach. | Culture medium, antibiotics, temperature. | [115] |
| E. nigrum | Terminalia arjuna | Digoxin | Regulating the heart rhythm and strengthening heart diffusion | One parameter at time approach. | Culture medium, temperature, elicitors, incubation time, pH, medium volume, inoculum age, inoculum size, gamma irradiation mutagenesis. | [113] |
| Penicillium mallochii | A beech tree bark from Balikesir, Turkey | Orange-red pigment | Decreasing in allergic responses to synthetic pigments | One parameter at time approach. | Culture medium, pH, temperature. | [116] |
| Tausonia pullulans | Vinca minor | Vincamine | Improvement of cerebrovascular and cognitive disorders and reduction the effects of certain types of stroke | Protoplasts optimization. | Incubation time, temperature, modulators, protoplast inactivation method (heat, ultraviolet, microwave, sodium nitrite, and diethyl sulfate). | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, J.A.; Barbosa, B.V.R.; Martins, B.d.A.; P. Guirlanda, C.; A. F. Moura, M. Use of the Versatility of Fungal Metabolism to Meet Modern Demands for Healthy Aging, Functional Foods, and Sustainability. J. Fungi 2020, 6, 223. https://doi.org/10.3390/jof6040223
Takahashi JA, Barbosa BVR, Martins BdA, P. Guirlanda C, A. F. Moura M. Use of the Versatility of Fungal Metabolism to Meet Modern Demands for Healthy Aging, Functional Foods, and Sustainability. Journal of Fungi. 2020; 6(4):223. https://doi.org/10.3390/jof6040223
Chicago/Turabian StyleTakahashi, Jacqueline A., Bianca V. R. Barbosa, Bruna de A. Martins, Christiano P. Guirlanda, and Marília A. F. Moura. 2020. "Use of the Versatility of Fungal Metabolism to Meet Modern Demands for Healthy Aging, Functional Foods, and Sustainability" Journal of Fungi 6, no. 4: 223. https://doi.org/10.3390/jof6040223
APA StyleTakahashi, J. A., Barbosa, B. V. R., Martins, B. d. A., P. Guirlanda, C., & A. F. Moura, M. (2020). Use of the Versatility of Fungal Metabolism to Meet Modern Demands for Healthy Aging, Functional Foods, and Sustainability. Journal of Fungi, 6(4), 223. https://doi.org/10.3390/jof6040223