1. Introduction
Biological aerosols are a subset of atmospheric particles consisting of both living and non-living organisms, including bacteria, viruses, pollens, molds, yeast-like fungi, and their metabolic products (e.g., mycotoxins) [
1,
2]. Most molds are not harmful to humans, however, previous studies, including ours, have shown that yeast-like fungi and spores of mold fungi are the etiological factors of many diseases, including allergy, pneumonia, bronchitis, neoplastic diseases, and type 1 diabetes [
3,
4,
5,
6]. The storage and sorting of organic waste, composting, agricultural production, food processing, and wastewater treatment systems emit large volumes of bioaerosols, which lead to significant exposure to biological factors [
7,
8,
9]. Studies have demonstrated that not only emergency and uncontrolled wastewater disposals but also outflows from treatment plants and heavy rains cause an increase in the number of microorganisms, such as mold and yeast-like fungi in coastal seawater and sand [
8,
9,
10,
11,
12]. Moreover, the mold and yeast-like fungi survive in salt seawater and in beach sand for many months [
13,
14,
15]. More than 30 years ago, Anderson (1979) discovered that pathogenic fungi, for instance,
Trichosporon cutaneum, Candida albicans, Microsporum gypseum, and
Trichophyton mentagrophytes, could survive in the sand for over a month [
16]. A similar study was conducted by other authors who demonstrated that several species of dermatophytes (
Epidermophyton floccosum, Microsporum canis, M. gypseum, T. mentagrophytes, T. rubrum), and
Scopulariopsis brevicaulis can survive in the sand from 25 to 360 days [
17,
18]. Therefore, Vogel et al. suggested that pathogenic yeast-like fungi found in seawater, sewage and beach sand could be a good additional mycological indicator in assessing the safety of marine bathing waters [
18].
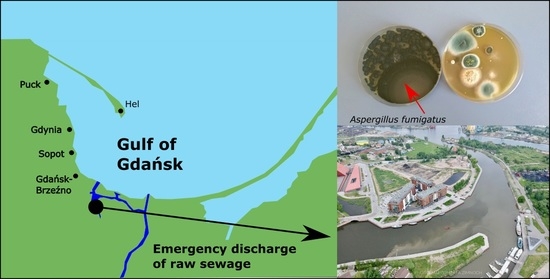
In terms of mycology, clean air and seawater are of key importance to the health of seaside town inhabitants. This is especially true of holiday resorts that dispose of treated sewage into seawaters. A typical example is seaside bathing areas located along the Gulf of Gdańsk—a bay in the south-eastern part of the Baltic Sea, located between Poland and Russia. Wastewater from the two largest sewage treatment plants is disposed of into the Gulf of Gdańsk [
19]. The first (Gdańsk-Wschód) disposes of sewage into the Gulf of Gdańsk to a distance of 2.5 km from the shoreline through a deep water collector, the second (Gdynia-Dębogórze) uses a collector over a distance of 2.3 km to dispose of wastewater to the Bay of Puck (western part of the Gulf of Gdańsk). What is more, from the 15
th to 18
th May 2018, there was an emergency raw sewage disposal into the Motława River, which flows into the Gulf of Gdańsk (
Figure 1). We have adopted a research hypothesis that emergency disposal of raw sewage into the Gulf of Gdańsk could have led to microbiological contamination of the water in the Gulf of Gdańsk and the local air. The preliminary results of our study showed an increase in the number of coliform bacteria and
Escherichia coli in the seawater and air as a result of emergency disposal of raw sewage to the Gulf of Gdańsk in 2018 [
7]. Thus far, the results of mycological tests performed after the disposal have not been presented. Therefore, the aim of this study was to determine the correlation between the meteorological factors and the number of molds and yeast-like fungi in the air in the five coastal towns in the years 2014–2017, and in 2018, after emergency disposal of raw of sewage to the Gdańsk Gulf.
4. Discussion
The analysis based on the PCA correlation of mold and yeast-like fungi detected in air samples in the seaside towns and meteorological factors showed a statistically significant relationship (
p < 0.00001). The principal component (PC1) was correlated with the air and water temperature, and wind speed explains 33.54% of the total variance. The second principal component (PC2) was correlated with the relative air humidity, air temperature, direction, and wind speed, and explains 27.23% of the total variance (
Table 2). Similar research results were obtained by Grinn-Gofron and Bosiacka [
28]. Their 4-year study showed that air temperature, dew point, relative humidity, and average wind speed had the greatest influence on the composition of spores in the air [
28]. Previous interdisciplinary studies too, including our research, showed that the direction and speed of the wind are one of the most important meteorological factors affecting the formation of aerosols at the water-air interface [
29,
30,
31,
32]. In our study, the important meteorological factors affecting airborne spore of
Aspergillus sp. and
Penicillium sp. concentrations were relative humidity and wind speed. The same results were noted by Grinn-Gofroń in Szczecin. The daily values of relative humidity and average wind speed were positively correlated for
p = 0.001 and
p = 0.01, respectively [
33]. In addition, the principal component (PC1) and (PC2) of
Cladosporium sp. was significantly correlated (
p < 0.00001) with the wind speed, air temperature, and relative humidity. In the same way, air temperature, wind speed, and relative humidity were associated with
Cladosporium spore dispersal in Morocco [
34]. In addition, our PCA statistical study of the seaside air of 2018 revealed that, compared to the number of fungi detected in the years 2014–2017, the highest number of mold and yeast-like fungi, after emergency disposal of sewage into the Gulf of Gdańsk, was detected in Hel, Sopot, and Gdańsk-Brzeźno (
Figure 2). We believe that the greater number of mold fungi in the air samples in these seaside towns could have been influenced not only by water and air temperature, wind speed, and humidity but also by the direction of the wind (SE) blowing from the Bay of Gdańsk. We suggest that it is advisable to inform residents about the potential health risk in the event of raw sewage disposal into the water.
On the other hand, in Gdynia, in 2018, neither mold nor yeast-like fungi were found compared to the period of 2014–2017. The lack of mold and yeast-like fungi in the air of Gdynia in 2018 could be caused by the direction of the wind blowing from the sea (NE). The Gulf of Gdańsk is partially separated from the Baltic Sea by the Hel Peninsula and the city of Hel. In Hel, the dominant wind direction is west, and in Gdynia, a north-east direction. In the city of Gdynia, due to the presence of the Hel Peninsula, the wind blowing from the direction of the sea is less frequently observed.
To our knowledge, no results of mycological studies on the quality of seaside air after emergency disposal of raw sewage are available. Therefore, the outcomes of 2018 were compared to the quality of air at the sewage treatment plant in order to indicate the likely origin of the species. Filamentous fungi of the genus
Aspergillus,
Cladosporium, and
Mucor and yeast-like fungi, for example,
Candida, were detected in domestic human and animal sewage [
35,
36,
37,
38]. Michałkiewicz et al. found that the majority of yeast-like fungi isolated from the air of the four wastewater treatment plants was
Candida [
39]. Other studies demonstrated that the following mold and yeast-like fungi were predominant:
Cladosporium sp.,
A. fumigatus, A. alternata, C. albicans, and
Rhodotorula sp. [
40,
41,
42,
43,
44]. Potentially pathogenic fungi, such as
Olpidium, Paecilomyces, Aspergillus, Rhodotorula, Penicillium, Candida, Synchytrium, Phyllosticta, and
Mucor have been detected in three wastewater treatment plants located in the Gauteng province of the Republic of South Africa [
45]. In Portuguese studies, mold fungi
Aspergillus,
Fusarium, and yeast-like fungi
Candida were found in beach sand contaminated with leaking toilet sewage [
12].
In turn, other researchers conducted a sanitary evaluation of sand and water from 16 beaches of São Paulo State, Brazil [
46]. Ninety-six samples each of wet and dry sand and seawater were collected and analyzed for fecal indicator bacteria. Correlation analysis indicated a significant relationship between fecal indicator densities in wet sand and seawater. There was a significant correlation between the densities of fecal coliforms and fecal streptococci for both types of sand, and this correlation was higher in wet sand. These data suggest the necessity of some criteria for microbiological control [
46]. Transmission of infectious diseases in terrestrial beach environments can occur via direct exposure to microbes found in sand or through the flux of microbes from water to sand within the swash or intertidal zone. In addition to direct exposure, sand can also serve as a vehicle for transferring pathogenic microbes to and from the adjacent water [
10].
Recent research suggests that being in and using the beach may be a risk factor for infectious diseases, thus monitoring of both seawater, beach sand, and coastal air is warranted [
7,
10,
46,
47].
In conclusion, the study results of 2018 indicate that untreated wastewater associated with emergency disposal to the Gulf of Gdańsk was a likely source of mold and yeast-like fungi in the seaside air. The analysis of PCA data demonstrated a statistically significant relationship between the meteorological factors and the number of mold and yeast-like fungi reported in the period of 2014–2017 and in 2018. Although failures of sewage treatment plant collectors, heavy rainfall, and floods occur quite often, they should not lead to an increase in the number of potentially pathogenic bacteria and mold and yeast-like fungi in the coastal seawater and air. Therefore, it is important to build new wastewater treatment plants, and expand and modernize the existing ones, thus that pathogenic microorganisms are effectively eliminated in the process of wastewater treatment. Moreover, tighter measures, including wastewater disinfection, should be introduced in wastewater treatment plants.