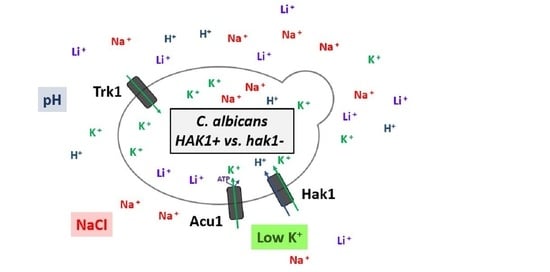
The Potassium Transporter Hak1 in Candida Albicans, Regulation and Physiological Effects at Limiting Potassium and under Acidic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Cultivation
2.2. Growth Assays
2.3. Cation Content and Transport
2.4. RNA Isolation and Reverse Transcription
2.5. Real-Time PCR
2.6. Statistics
3. Results and Discussion
3.1. Potassium Requirements and Transport Characteristics in HAK1+ and hak1− Candida albicans
3.2. Transcriptional Regulation of the C. albicans Plasma Membrane Potassium Transporters
3.3. Role of Hak1 in Tolerance to the Toxic Lithium Cation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 135–189. ISBN 9780123849052. [Google Scholar]
- Ramos, J.; Ariño, J.; Sychrová, H. Alkali-metal-cation influx and efflux systems in nonconventional yeast species. FEMS Microbiol. Lett. 2011, 317, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariño, J.; Ramos, J.; Sychrova, H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast 2019, 36, 177–193. [Google Scholar] [CrossRef]
- Benito, B.; Garciadeblás, B.; Schreier, P.; Rodríguez-Navarro, A. Novel p-type ATPases mediate high-affinity potassium or sodium uptake in fungi. Eukaryot. Cell 2004, 3, 359–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elicharová, H.; Hušeková, B.; Sychrová, H. Three Candida albicans potassium uptake systems differ in their ability to provide Saccharomyces cerevisiae trk1trk2 mutants with necessary potassium. FEMS Yeast Res. 2016, 16, 39. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.L.; Sychrova, H.; Ramos, J. Monovalent cations regulate expression and activity of the Hak1 potassium transporter in Debaryomyces hansenii. Fungal Genet. Biol. 2011, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, E.; Álvarez, M.C.; Martín, Y.; Siverio, J.M.; Ramos, J. K+ uptake systems in the yeast Hansenula polymorpha. Transcriptional and post-translational mechanisms involved in high-affinity K+ transporter regulation. Fungal Genet. Biol. 2012, 49, 755–763. [Google Scholar] [CrossRef]
- Haro, R.; Sainz, L.; Rubio, F.; Rodríguez-Navarro, A. Cloning of two genes encoding potassium transporters in Neurospora crassa and expression of the corresponding cDNAs in Saccharomyces cerevisiae. Mol. Microbiol. 1999, 31, 511–520. [Google Scholar] [CrossRef]
- Rivetta, A.; Allen, K.E.; Slayman, C.W.; Slayman, C.L. Coordination of K+ Transporters in Neurospora: TRK1 Is Scarce and Constitutive, while HAK1 Is Abundant and Highly Regulated. Eukaryot. Cell 2013, 12, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieves-Cordones, M.; Alemán, F.; Martínez, V.; Rubio, F. K+ uptake in plant roots. The systems involved, their regulation and parallels in other organisms. J. Plant Physiol. 2014, 171, 688–695. [Google Scholar] [CrossRef]
- Santa-María, G.E.; Oliferuk, S.; Moriconi, J.I. KT-HAK-KUP transporters in major terrestrial photosynthetic organisms: A twenty years tale. J. Plant Physiol. 2018, 226, 77–90. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Zakharyan, E.; Trchounian, A. K+influx by Kup in Escherichia coli is accompanied by a decrease in H+efflux. FEMS Microbiol. Lett. 2001, 204, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Bañuelos, M.; Klein, R.; Alexander-Bowman, S.; Rodríguez-Navarro, A. A potassium transporter of the yeast Schwanniomyces occidentalis homologous to the Kup system of Escherichia coli has a high concentrative capacity. EMBO J. 1995, 14, 3021–3027. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.; Blatt, M.R.; Slayman, C.L. A potassium-proton symport in Neurospora crassa. J. Gen. Physiol. 1986, 87, 649–674. [Google Scholar] [CrossRef] [Green Version]
- Maathuis, F.J.; Sanders, D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1994, 91, 9272–9276. [Google Scholar] [CrossRef] [Green Version]
- Benito, B.; Garciadeblás, B.; Fraile-Escanciano, A.; Rodríguez-Navarro, A. Potassium and sodium uptake systems in fungi. The transporter diversity of Magnaporthe oryzae. Fungal Genet. Biol. 2011, 48, 812–822. [Google Scholar] [CrossRef]
- Schulze, J.; Sonnenborn, U. Yeasts in the gut: From commensals to infectious agents. Dtsch. Arztebl. Int. 2009, 106, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Witherden, E.A.; Shoaie, S.; Hall, R.A.; Moyes, D.L. The Human Mucosal Mycobiome and Fungal Community Interactions. J. Fungi 2017, 3, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baev, D.; Rivetta, A.; Vylkova, S.; Sun, J.N.; Zeng, G.-F.; Slayman, C.L.; Edgerton, M. The TRK1 Potassium Transporter Is the Critical Effector for Killing of Candida albicans by the Cationic Protein, Histatin 5. J. Biol. Chem. 2004, 279, 55060–55072. [Google Scholar] [CrossRef] [Green Version]
- Miranda, M.; Bashi, E.; Vylkova, S.; Edgerton, M.; Slayman, C.; Rivetta, A. Conservation and dispersion of sequence and function in fungal TRK potassium transporters: Focus on Candida albicans. FEMS Yeast Res. 2009, 9, 278–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Castilla, F.J.; Bieber, J.; Caro, G.; Michán, C.; Sychrova, H.; Ramos, J. Regulation and activity of CaTrk1, CaAcu1 and CaHak1, the three plasma membrane potassium transporters in Candida albicans. Biochim. Biophys. Acta BBA Biomembr. 2021, 1863, 183486. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.M.; French, S.; Kohn, L.A.; Chen, V.; Johnson, A.D. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 2010, 42, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Prista, C.; Almagro, A.; Loureiro-Dias, M.C.; Ramos, J. Physiological basis for the high salt tolerance of Debaryomyces hansenii. Appl. Environ. Microbiol. 1997, 63, 4005–4009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, J.; Haro, R.; Rodríguez-Navarro, A. Regulation of potassium fluxes in Saccharomyces cerevisiae. Biochim. Biophys. Acta BBA Biomembr. 1990, 1029, 211–217. [Google Scholar] [CrossRef]
- Navarrete, C.; Petrezsélyová, S.; Barreto, L.; Martínez, J.L.; Zahrádka, J.; Ariño, J.; Sychrová, H.; Ramos, J. Lack of main K+ uptake systems in Saccharomyces cerevisiae cells affects yeast performance in both potassium-sufficient and potassium-limiting conditions. FEMS Yeast Res. 2010, 10, 508–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabov, A. Plant KT/KUP/HAK Potassium Transporters: Single Family—Multiple Functions. Ann. Bot. 2007, 99, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Tascón, I.; Sousa, J.S.; Corey, R.A.; Mills, D.J.; Griwatz, D.; Aumüller, N.; Mikusevic, V.; Stansfeld, P.J.; Vonck, J.; Hänelt, I. Structural basis of proton-coupled potassium transport in the KUP family. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Madrid, R.; Rodríguez-Navarro, A. Individual functions of the HAK and TRK potassium transporters of Schwanniomyces occidentalis. Mol. Microbiol. 2002, 37, 671–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Navarro, A. Potassium transport in fungi and plants. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 2000, 1469, 1–30. [Google Scholar] [CrossRef]
- Véry, A.-A.; Nieves-Cordones, M.; Daly, M.; Khan, I.; Fizames, C.; Sentenac, H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014, 171, 748–769. [Google Scholar] [CrossRef]
- Nieves-Cordones, M.; Ródenas, R.; Chavanieu, A.; Rivero, R.M.; Martinez, V.; Gaillard, I.; Rubio, F. Uneven HAK/KUP/KT Protein Diversity Among Angiosperms: Species Distribution and Perspectives. Front. Plant Sci. 2016, 7, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, T.; Federspiel, N.A.; Chibana, H.; Dungan, J.; Kalman, S.; Magee, B.B.; Newport, G.; Thorstenson, Y.R.; Agabian, N.; Magee, P.T.; et al. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. USA 2004, 101, 7329–7334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, M.J.; Luyten, K.; Ramos, J. The capacity to transport potassium influences sodium tolerance in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1996, 135, 157–160. [Google Scholar] [CrossRef]





| pH | HAK1 | Normal K+ Cells | K+ -Starved Cells | ||
|---|---|---|---|---|---|
| Vmax | Km | Vmax | Km | ||
| (nmol∙mg−1∙min−1) | (mM) | (nmol∙mg−1∙min−1) | (mM) | ||
| 3.0 | + | 0.63 ± 0.20 | 1.63 ± 0.55 | 11.49 ± 0.20 | 0.09 ± 0.02 |
| − | 0.45 ± 0.22 | 1.88 ± 0.42 | 0.89 ± 0.07 *** | 3.75 ± 0.05 *** | |
| 4.5 | + | 0.91 ± 0.12 | 0.95 ± 0.04 | 13.71 ± 0.58 | 0.03 ± 0.02 |
| − | 0.74 ± 0.09 | 0.87 ± 0.05 | 3.69 ± 0.43 *** | 0.59 ± 0.06 *** | |
| 5.8 | + | 1.96 ± 0.33 | 0.25 ± 0.02 | 9.79 ± 0.43 | 0.09 ± 0.02 |
| − | 1.34 ± 0.20 * | 0.32 ± 0.03 | 5.57 ± 1.25 ** | 0.55 ± 0.17 ** | |
| 7.5 | + | 2.68 ± 0.27 | 0.36 ± 0.03 | 9.92 ± 1.65 | 0.28 ± 0.08 |
| − | 2.01 ± 0.11 * | 0.40 ± 0.07 | 7.89 ± 1.79 | 0.74 ± 0.25 ** | |
| pH | HAK1 | 0.30 M LiCl | |
|---|---|---|---|
| Lithium (nmol∙mg−1) | Potassium (nmol∙mg−1) | ||
| 3.0 | + | 13.9 ± 0.4 | 242.7 ± 12.5 |
| − | 14.8 ± 0.5 | 185.9 ± 10.4 ** | |
| 7.5 | + | 13.1 ± 3.8 | 126.3 ± 12.8 |
| − | 14.3 ± 4.6 | 122.3 ± 7.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Castilla, F.J.; Rodríguez-Castro, E.; Michán, C.; Ramos, J. The Potassium Transporter Hak1 in Candida Albicans, Regulation and Physiological Effects at Limiting Potassium and under Acidic Conditions. J. Fungi 2021, 7, 362. https://doi.org/10.3390/jof7050362
Ruiz-Castilla FJ, Rodríguez-Castro E, Michán C, Ramos J. The Potassium Transporter Hak1 in Candida Albicans, Regulation and Physiological Effects at Limiting Potassium and under Acidic Conditions. Journal of Fungi. 2021; 7(5):362. https://doi.org/10.3390/jof7050362
Chicago/Turabian StyleRuiz-Castilla, Francisco J., Elisa Rodríguez-Castro, Carmen Michán, and José Ramos. 2021. "The Potassium Transporter Hak1 in Candida Albicans, Regulation and Physiological Effects at Limiting Potassium and under Acidic Conditions" Journal of Fungi 7, no. 5: 362. https://doi.org/10.3390/jof7050362
APA StyleRuiz-Castilla, F. J., Rodríguez-Castro, E., Michán, C., & Ramos, J. (2021). The Potassium Transporter Hak1 in Candida Albicans, Regulation and Physiological Effects at Limiting Potassium and under Acidic Conditions. Journal of Fungi, 7(5), 362. https://doi.org/10.3390/jof7050362