Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems
Abstract
:1. Introduction
2. Beneficial Effects of Trichoderma in Agroecosystems
3. Biosynthesis of Nanoparticles by Trichoderma Genus
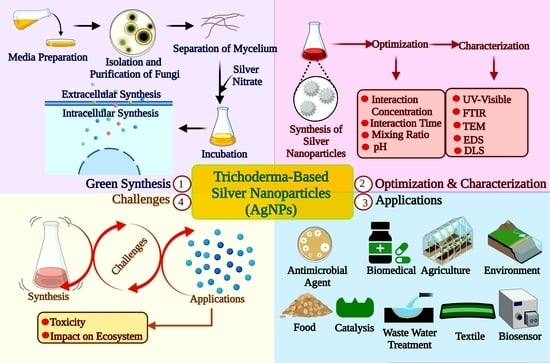
3.1. Silver Nanoparticles
3.2. Zinc Oxide Nanoparticles
3.3. Copper Nanoparticles
3.4. Selenium Nanoparticles
3.5. Other Nanoparticles
4. Production of Metal NPs by Hypocrea
5. Toxicity
6. Understanding the Mechanism of Synthesis of Trichogenic NPs
6.1. Bioactive Metabolites
6.2. Enzymes
7. Applications of Trichoderma-Mediated NPs in the Agri-Food Sector
7.1. Antifungal Activity
7.2. Antibacterial Activity
7.3. Plant Growth Promotion
7.4. Trichoderma-Based Nanobioremediation
7.5. Miscellaneous Advantages and Disadvantages of Trichogenic Nanoparticles
8. Challenges
9. Future Trends
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghuthaymi, M.A.; Rajkuberan, C.; Rajiv, P.; Kalia, A.; Bhardwaj, K.; Bhardwaj, P.; Abd-Elsalam, K.A.; Valis, M.; Kuca, K. Nanohybrid antifungals for control of plant diseases: Current status and future perspectives. J. Fungi 2021, 7, 48. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-Elsalam, K.A.; Khaleil, M.M. Bacillus megaterium-Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma harzianum-Mediated ZnO Nanoparticles: A Green Tool for Controlling Soil-Borne Pathogens in Cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef]
- Guilger, M.; Pasquoto-Stigliani, T.; Bilesky-Jose, N.; Grillo, R.; Abhilash, P.C.; Fraceto, L.F.; De Lima, R. Biogenic silver nanoparticles based on Trichoderma harzianum: Synthesis, characterization, toxicity evaluation and biological activity. Sci. Rep. 2017, 7, 44421. [Google Scholar] [CrossRef] [PubMed]
- Mahawar, H.; Prasanna, R. Prospecting the interactions of nanoparticles with beneficial microorganisms for developing green technologies for agriculture. Environ. Nanotechnol. Monit. Manag. 2018, 10, 477–485. [Google Scholar] [CrossRef]
- Chauhan, A.; Anand, J.; Parkash, V.; Rai, N. Biogenic synthesis: A sustainable approach for nanoparticles synthesis mediated by fungi. Inorg. Nano-Metal Chem. 2022, 1–14. [Google Scholar] [CrossRef]
- Misra, M.; Chattopadhyay, S.; Sachan, A.; Sachan, S.G. Microbially synthesized nanoparticles and their applications in environmental clean-up. Environ. Technol. Rev. 2022, 11, 18–32. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- El-Rahman, A.A.E.-M.A.; El-Shafei, S.M.A.E.-A.; Ivanova, E.V.; Fattakhova, A.N.; Pankova, A.V.; El-Shafei, M.A.E.-A.; El-Morsi, E.-M.A.E.-F.; Alimova, F.K. Cytotoxicity of Trichoderma spp. Cultural Filtrate Against Human Cervical and Breast Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2014, 15, 7229–7234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.P.; Handa, R.; Manchanda, G. Nanoparticles in sustainable agriculture: An emerging opportunity. J. Control. Release 2021, 329, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Elegbede, J.A.; Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Oladipo, I.C.; Aina, D.A.; Beukes, L.S.; Gueguim-Kana, E.B. Biofabrication of Gold Nanoparticles Using Xylanases Through Valorization of Corncob by Aspergillus niger and Trichoderma longibrachiatum: Antimicrobial, Antioxidant, Anticoagulant and Thrombolytic Activities. Waste Biomass Valoriz. 2020, 11, 781–791. [Google Scholar] [CrossRef]
- Ramírez-Valdespino, C.A.; Orrantia-Borunda, E. Trichoderma and Nanotechnology in Sustainable Agriculture: A Review. Front. Fungal Biol. 2021, 2. [Google Scholar] [CrossRef]
- Zaki, S.A.; Ouf, S.A.; Abd-Elsalam, K.A.; Asran, A.A.; Hassan, M.M.; Kalia, A.; Albarakaty, F.M. Trichogenic Silver-Based Nanoparticles for Suppression of Fungi Involved in Damping-Off of Cotton Seedlings. Microorganisms 2022, 10, 344. [Google Scholar] [CrossRef] [PubMed]
- Konappa, N.; Udayashankar, A.C.; Dhamodaran, N.; Krishnamurthy, S.; Jagannath, S.; Uzma, F.; Pradeep, C.K.; De Britto, S.; Chowdappa, S.; Jogaiah, S. Ameliorated antibacterial and antioxidant properties by Trichoderma harzianum mediated green synthesis of silver nanoparticles. Biomolecules 2021, 11, 535. [Google Scholar] [CrossRef]
- Madbouly, A.K. Biodiversity of Genus Trichoderma and Their Potential Applications. In Industrially Important Fungi for Sustainable Development. Fungal Biology; Abdel-Azeem, A.M., Yadav, A.N., Yadav, N., Usmani, Z., Eds.; Springer: Cham, Switzerland, 2021; pp. 429–460. [Google Scholar]
- Bitas, V.; Kim, H.-S.; Bennett, J.W.; Kang, S. Sniffing on Microbes: Diverse Roles of Microbial Volatile Organic Compounds in Plant Health. Mol. Plant-Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Bar, M. Plant Immunity, Priming, and Systemic Resistance as Mechanisms for Trichoderma spp. Biocontrol. In Trichoderma; Sharma, A., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 81–110. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Nakkeeran, S.; Rajamanickam, S.; Vanthana, M.; Renukadevi, P.; Muthamilan, M. Harnessing the Perception of Trichoderma Signal Molecules in Rhizosphere to Improve Soil Health and Plant Health. In Trichoderma; Springer: Singapore, 2020; pp. 61–79. [Google Scholar]
- Chakraborty, B.N.; Chakraborty, U.; Sunar, K. Induced Immunity Developed by Trichoderma species in Plants. In Trichoderma; Springer: Singapore, 2020; pp. 125–147. [Google Scholar]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Meher, J.; Rajput, R.S.; Bajpai, R.; Teli, B.; Sarma, B.K. Trichoderma: A Globally Dominant Commercial Biofungicide. In Trichoderma: Agricultural Applications and Beyond; Springer: Cham, Switzerland, 2020; pp. 195–208. [Google Scholar]
- Ram, R.M.; Vaishnav, A.; Singh, H.B. Trichoderma spp.: Expanding Potential beyond Agriculture. In Trichoderma: Agricultural Applications and Beyond; Springer: Cham, Switzerland, 2020; pp. 351–367. [Google Scholar]
- Weber, R.W.S.; Ridderbusch, D.C.; Anke, H. 2,4,6-Trinitrotoluene (TNT) tolerance and biotransformation potential of microfungi isolated from TNT-contaminated soil. Mycol. Res. 2002, 106, 336–344. [Google Scholar] [CrossRef]
- Nazifa, T.H.; Bin Ahmad, M.A.; Hadibarata, T.; Salmiati; Aris, A. Bioremediation of diesel oil spill by filamentous fungus Trichoderma reesei H002 in aquatic environment. Int. J. Integr. Eng. 2018, 10, 14–19. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Caniani, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K. Bioremediation mechanism and potential of copper by actively growing fungus Trichoderma lixii CR700 isolated from electroplating wastewater. J. Environ. Manag. 2021, 277, 111370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, X.; Huo, Y.; Xiao, Y. Trichoderma-Inoculation and Mowing Synergistically Altered Soil Available Nutrients, Rhizosphere Chemical Compounds and Soil Microbial Community, Potentially Driving Alfalfa Growth. Front. Microbiol. 2019, 9, 3241. [Google Scholar] [CrossRef] [PubMed]
- Molla, A.H.; Manjurul Haque, M.; Amdadul Haque, M.; Ilias, G.N.M. Trichoderma-Enriched Biofertilizer Enhances Production and Nutritional Quality of Tomato (Lycopersicon esculentum Mill.) and Minimizes NPK Fertilizer Use. Agric. Res. 2012, 1, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Singh, U.B.; Malviya, D.; Wasiullah; Singh, S.; Pradhan, J.K.; Singh, B.P.; Roy, M.; Imram, M.; Pathak, N.; Baisyal, B.M.; et al. Bio-protective microbial agents from rhizosphere eco-systems trigger plant defense responses provide protection against sheath blight disease in rice (Oryza sativa L.). Microbiol. Res. 2016, 192, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Willian, G.A. Biodegradation of Chlorpyrifos by Whole Cells of Marine-Derived Fungi Aspergillus sydowii and Trichoderma sp. J. Microb. Biochem. Technol. 2015, 7, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Podbielska, M.; Kus-Liśkiewicz, M.; Jagusztyn, B.; Piechowicz, B.; Sadło, S.; Słowik-Borowiec, M.; Twarużek, M.; Szpyrka, E. Influence of Bacillus subtilis and Trichoderma harzianum on Penthiopyrad Degradation under Laboratory and Field Studies. Molecules 2020, 25, 1421. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Yuan, X.; Li, Y.; Wang, X.; Chen, J. The pathway of 2,2-dichlorovinyl dimethyl phosphate (DDVP) degradation by Trichoderma atroviride strain T23 and characterization of a paraoxonase-like enzyme. Appl. Microbiol. Biotechnol. 2019, 103, 8947–8962. [Google Scholar] [CrossRef]
- Wu, Q.; Ni, M.; Wang, G.; Liu, Q.; Yu, M.; Tang, J. Omics for understanding the tolerant mechanism of Trichoderma asperellum TJ01 to organophosphorus pesticide dichlorvos. BMC Genom. 2018, 19, 596. [Google Scholar] [CrossRef]
- Salwan, R.; Rialch, N.; Sharma, V. Bioactive Volatile Metabolites of Trichoderma: An overview. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Springer: Singapore, 2019; pp. 87–111. [Google Scholar]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of Silver Nanoparticles by Fungus Trichoderma reesei (A Route for Large-Scale Production of AgNPs). Insciences J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Special Issue: Fungal Nanotechnology. J. Fungi 2021, 7, 583. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, L.; Riaz Rajoka, M.S.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef]
- Ayad, F.; Matallah-Boutiba, A.; Rouane–Hacene, O.; Bouderbala, M.; Boutiba, Z. Tolerance of Trichoderma sp. to Heavy Metals and its Antifungal Activity in Algerian Marine Environment. J. Pure Appl. Microbiol. 2018, 12, 855–870. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; Lima, R. de Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green synthesis of highly stabilized nanocrystalline silver particles by a non-pathogenic and agriculturally important fungus T. asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef] [PubMed]
- Prameela Devi, T.; Kulanthaivel, S.; Kamil, D.; Borah, J.L.; Prabhakaran, N.; Srinivasa, N. Biosynthesis of silver nanoparticles from Trichoderma species. Indian J. Exp. Biol. 2013, 51, 543–547. [Google Scholar]
- Ahluwalia, V.; Kumar, J.; Sisodia, R.; Shakil, N.A.; Walia, S. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crops Prod. 2014, 55, 202–206. [Google Scholar] [CrossRef]
- Sundaravadivelan, C.; Padmanabhan, M.N. Effect of mycosynthesized silver nanoparticles from filtrate of Trichoderma harzianum against larvae and pupa of dengue vector Aedes aegypti L. Environ. Sci. Pollut. Res. 2014, 21, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Elgorban, A.M.; Al-Rahmah, A.N.; Sayed, S.R.; Hirad, A.; Mostafa, A.A.F.; Bahkali, A.H. Antimicrobial activity and green synthesis of silver nanoparticles using Trichoderma viride. Biotechnol. Biotechnol. Equip. 2016, 30, 299–304. [Google Scholar] [CrossRef] [Green Version]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Saravanakumar, K.; Wang, M.-H. Trichoderma based synthesis of anti-pathogenic silver nanoparticles and their characterization, antioxidant and cytotoxicity properties. Microb. Pathog. 2018, 114, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Adebayo-Tayo, B.; Ogunleye, G.; Ogbole, O. Biomedical application of greenly synthesized silver nanoparticles using the filtrate of Trichoderma viride: Anticancer and immunomodulatory potentials. Polym. Med. 2020, 49, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Gemishev, O.T.; Panayotova, M.I.; Mintcheva, N.N.; Djerahov, L.P.; Tyuliev, G.T.; Gicheva, G.D. A green approach for silver nanoparticles preparation by cell-free extract from Trichoderma reesei fungi and their characterization. Mater. Res. Express 2019, 6, 095040. [Google Scholar] [CrossRef]
- Omran, B.A.; Nassar, H.N.; Younis, S.A.; Fatthallah, N.A.; Hamdy, A.; El-Shatoury, E.H.; El-Gendy, N.S. Physiochemical properties of Trichoderma longibrachiatum DSMZ 16517-synthesized silver nanoparticles for the mitigation of halotolerant sulphate-reducing bacteria. J. Appl. Microbiol. 2019, 126, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, A.; Nada, A.A.; O’Donovan, A.; Kumar Thakur, V.; Elkelish, A. Mycogenic Silver Nanoparticles from Endophytic Trichoderma atroviride with Antimicrobial Activity. J. Renew. Mater. 2020, 8, 171–185. [Google Scholar] [CrossRef]
- Hirpara, D.G.; Gajera, H.P. Green synthesis and antifungal mechanism of silver nanoparticles derived from chitin- induced exometabolites of Trichoderma interfusant. Appl. Organomet. Chem. 2020, 34, e5407. [Google Scholar] [CrossRef]
- Ramos, M.M.; dos Morais, E.S.; da Sena, I.S.; Lima, A.L.; de Oliveira, F.R.; de Freitas, C.M.; Fernandes, C.P.; de Carvalho, J.C.T.; Ferreira, I.M. Silver nanoparticle from whole cells of the fungi Trichoderma spp. isolated from Brazilian Amazon. Biotechnol. Lett. 2020, 42, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Tomah, A.A.; Alamer, I.S.A.; Li, B.; Zhang, J.-Z. Mycosynthesis of Silver Nanoparticles Using Screened Trichoderma Isolates and Their Antifungal Activity against Sclerotinia sclerotiorum. Nanomaterials 2020, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Jeevithan, E.; Hu, X.; Chelliah, R.; Oh, D.-H.; Wang, M.-H. Enhanced anti-lung carcinoma and anti-biofilm activity of fungal molecules mediated biogenic zinc oxide nanoparticles conjugated with β-D-glucan from barley. J. Photochem. Photobiol. B Biol. 2020, 203, 111728. [Google Scholar] [CrossRef] [PubMed]
- Shobha, B.; Lakshmeesha, T.R.; Ansari, M.A.; Almatroudi, A.; Alzohairy, M.A.; Basavaraju, S.; Alurappa, R.; Niranjana, S.R.; Chowdappa, S. Mycosynthesis of ZnO Nanoparticles Using Trichoderma spp. Isolated from Rhizosphere Soils and Its Synergistic Antibacterial Effect against Xanthomonas oryzae pv. oryzae. J. Fungi 2020, 6, 181. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Shanmugam, S.; Varukattu, N.B.; MubarakAli, D.; Kathiresan, K.; Wang, M.-H. Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J. Photochem. Photobiol. B Biol. 2019, 190, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Ahmadi-Nouraldinvand, F.; Afrouz, M.; Elias, S.G.; Eslamian, S. Green synthesis of copper nanoparticles extracted from guar seedling under Cu heavy-metal stress by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Escherichia coli. Environ. Earth Sci. 2022, 81, 54. [Google Scholar] [CrossRef]
- Natesan, K.; Ponmurugan, P.; Gnanamangai, B.M.; Manigandan, V.; Joy, S.P.J.; Jayakumar, C.; Amsaveni, G. Biosynthesis of silica and copper nanoparticles from Trichoderma, Streptomyces and Pseudomonas spp. evaluated against collar canker and red root-rot disease of tea plants. Arch. Phytopathol. Plant Prot. 2021, 54, 56–85. [Google Scholar] [CrossRef]
- Nandini, B.; Hariprasad, P.; Prakash, H.S.; Shetty, H.S.; Geetha, N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerospora graminicola in pearl millet. Sci. Rep. 2017, 7, 2612. [Google Scholar] [CrossRef]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control 2019, 106, 106748. [Google Scholar] [CrossRef]
- Diko, C.S.; Zhang, H.; Lian, S.; Fan, S.; Li, Z.; Qu, Y. Optimal synthesis conditions and characterization of selenium nanoparticles in Trichoderma sp. WL-Go culture broth. Mater. Chem. Phys. 2020, 246, 122583. [Google Scholar] [CrossRef]
- Mishra, A.; Kumari, M.; Pandey, S.; Chaudhry, V.; Gupta, K.C.; Nautiyal, C.S. Biocatalytic and antimicrobial activities of gold nanoparticles synthesized by Trichoderma sp. Bioresour. Technol. 2014, 166, 235–242. [Google Scholar] [CrossRef]
- Boruah, S.; Dutta, P. Fungus mediated biogenic synthesis and characterization of chitosan nanoparticles and its combine effect with Trichoderma asperellum against Fusarium oxysporum, Sclerotium rolfsii and Rhizoctonia solani. Indian Phytopathol. 2021, 74, 81–93. [Google Scholar] [CrossRef]
- El-Gazzar, N.; Almanaa, T.N.; Reda, R.M.; El Gaafary, M.N.; Rashwan, A.A.; Mahsoub, F. Assessment the using of silica nanoparticles (SiO2NPs) biosynthesized from rice husks by Trichoderma harzianum MF780864 as water lead adsorbent for immune status of Nile tilapia (Oreochromis niloticus). Saudi J. Biol. Sci. 2021, 28, 5119–5130. [Google Scholar] [CrossRef] [PubMed]
- Kareem, S.; Adeleye, T.; Ojo, R. Effects of pH, temperature and agitation on the biosynthesis of iron nanoparticles produced by Trichoderma species. IOP Conf. Ser. Mater. Sci. Eng. 2020, 805, 012036. [Google Scholar] [CrossRef]
- Bilesky-José, N.; Maruyama, C.; Germano-Costa, T.; Campos, E.; Carvalho, L.; Grillo, R.; Fraceto, L.F.; de Lima, R. Biogenic α-Fe2O3 Nanoparticles Enhance the Biological Activity of Trichoderma against the Plant Pathogen Sclerotinia sclerotiorum. ACS Sustain. Chem. Eng. 2021, 9, 1669–1683. [Google Scholar] [CrossRef]
- Chaverri, P.; Samuels, G.J. Hypocrea lixii, the teleomorph of Trichoderma harzianum. Mycol. Prog. 2002, 1, 283–286. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Lepre, L.F.; Ando, R.A.; Oller do Nascimento, C.A.; Corrêa, B. Biosynthesis and Uptake of Copper Nanoparticles by Dead Biomass of Hypocrea lixii Isolated from the Metal Mine in the Brazilian Amazon Region. PLoS ONE 2013, 8, e80519. [Google Scholar] [CrossRef] [PubMed]
- Salvadori, M.R.; Ando, R.A.; Oller Nascimento, C.A.; Corrêa, B. Extra and Intracellular Synthesis of Nickel Oxide Nanoparticles Mediated by Dead Fungal Biomass. PLoS ONE 2015, 10, e0129799. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Sajjad, W.; Wu, F.; Bibi, N.; Shah, K.; Yali, Z.; Wang, W. Green synthesis of silver nanoparticles and their shortcomings, animal blood a potential source for silver nanoparticles: A review. J. Hazard. Mater. Adv. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Lankveld, D.P.K.; Oomen, A.G.; Krystek, P.; Neigh, A.; de Jong, A.T.; Noorlander, C.W.; Van Eijkeren, J.C.H.; Geertsma, R.E.; De Jong, W.H. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials 2010, 31, 8350–8361. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Das, S.K.; Marsili, E. A green chemical approach for the synthesis of gold nanoparticles: Characterization and mechanistic aspect. Rev. Environ. Sci. Bio/Technol. 2010, 9, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Benítez, T.; Rincón, A.M.; Limón, M.C.; Codón, A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004, 7, 249–260. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Oksanen, T.; Pere, J.; Paavilainen, L.; Buchert, J.; Viikari, L. Treatment of recycled kraft pulps with Trichoderma reesei hemicellulases and cellulases. J. Biotechnol. 2000, 78, 39–48. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A.; Vasil’kov, A.Y.; Said-Galiev, E.E.; Rubina, M.S.; Khokhlov, A.R.; Naumkin, A.V.; Shtykova, E.V.; Alghuthaymi, M.A. Bimetallic blends and chitosan nanocomposites: Novel antifungal agents against cotton seedling damping-off. Eur. J. Plant Pathol. 2018, 151, 57–72. [Google Scholar] [CrossRef]
- Sawake, M.M.; Moharil, M.P.; Ingle, Y.V.; Jadhav, P.V.; Ingle, A.P.; Khelurkar, V.C.; Paithankar, D.H.; Bathe, G.A.; Gade, A.K. Management of Phytophthora parasitica causing gummosis in citrus using biogenic copper oxide nanoparticles. J. Appl. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Gemishev, O.; Panayotova, M.; Gicheva, G.; Mintcheva, N. Green Synthesis of Stable Spherical Monodisperse Silver Nanoparticles Using a Cell-Free Extract of Trichoderma reesei. Materials 2022, 15, 481. [Google Scholar] [CrossRef]
- Pandey, S.; Shahid, M.; Srivastava, M.; Singh, A.; Kumar, V.; Trivedi, S.; Srivastava, Y.K. Biosynthesis of SILVER nanoparticles by Trichoderma harzianum and its effect on the germination, growth and yield of tomato plant. J. Pure Appl. Microbiol. 2015, 9, 3335–3342. [Google Scholar]
- Shelar, G.B.; Chavan, A.M. Myco-synthesis of silver nanoparticles from Trichoderma harzianum and its impact on germination status of oil seed. Biolife 2015, 3, 109–113. [Google Scholar] [CrossRef]
- Bhadwal, A.S.; Tripathi, R.M.; Gupta, R.K.; Kumar, N.; Singh, R.P.; Shrivastav, A. Biogenic synthesis and photocatalytic activity of CdS nanoparticles. RSC Adv. 2014, 4, 9484. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, characterization and antifungal activity of chemically and fungal-produced silver nanoparticles against Trichophyton rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakya, M.; Rene, E.R.; Nancharaiah, Y.V.; Lens, P.N.L. Fungal-Based Nanotechnology for Heavy Metal Removal. In Nanotechnology, Food Security and Water Treatment; Springer: Berlin/Heidelberg, Germany, 2018; pp. 229–253. [Google Scholar]





| Trichoderma Species | NPs | Size | Shape | Application | References |
|---|---|---|---|---|---|
| T. asperellum | AgNPs | 13–18 nm | crystalline nature | Biomolecular detection | [44] |
| T. reesei | AgNPs | 5–50 nm | variable morphology | Preparing many nanostructured materials and devices | [39] |
| T. virens | AgNPs | 8–60 nm | round and uniform in shape | Crop protection | [45] |
| T. harzianum | AgNPs | 10–51 nm | face centered cubic symmetry particles | Antioxidant properties and antibacterial activity | [46] |
| T. harzianum | AgNPs | 10–20 nm | oval shaped, crystalline in nature | Mosquito control | [47] |
| T. viride | AgNPs | 1–50 nm | globular particles | Antibacterial effect against human pathogenic bacteria | [48] |
| T. harzianum | AgNPs | 20–30 nm | spherical | Control of S. sclerotiorum | [4] |
| T. longibrachiatum | AgNPs | 5–25 nm | spherical | Control of many phytopathogenic fungi | [49] |
| T. atroviride | AgNPs | 15–25 nm | anisotropic structural | Antioxidant and antibacterial against clinical pathogens | [50] |
| T. harzianum | AgNP-TS | 57.02 ± 1.75 nm | different characteristics | Control of S. sclerotiorum | [43] |
| AgNP-T | 81.84 ± 0.67 nm | ||||
| T. reesei | AgNPs | 1–4 nm 15–25 nm | crystal phase | carriers of biologically active molecules | [52] |
| T. longibrachiatum DSMZ 16517 | AgNPs | 5–11 ± 0·5 nm | spherical, triangular, and cuboid | Control of industrial microbes | [53] |
| Trichoderma sp. | AgNPs | 14–25 nm | round | Antibacterial | [56] |
| T. virens HZA14 | AgNPs | 5–50 nm | spherical and oval with smooth surfaces | Control of S. sclerotiorum | [57] |
| T. atroviride | AgNPs | 10–15 nm | spherical | Control of pathogenic bacteria and fungi | [54] |
| T. fusant Fu21 | AgNPs | 59.66 ± 4.18 nm | spherical | Control of S. sclerotiorum | [55] |
| T. harzianum | AgNPs | 72 nm | cubic crystal structure | Antioxidant properties and antibacterial activity | [15] |
| Trichoderma spp. co-culture | ZnONPs | 12–35 nm | crystal structure | Control of Bacterial Leaf Blight causative in rice | [59] |
| T. harzianum (SKCGW009) | ZnONPs | 30.34 nm | spherical | Antibacterial activity enhanced roundworm growth | [58] |
| T. harzianum | ZnONPs | 8–23 nm | hexagonal, spherical and rod | fungicidal action against three soil–cotton pathogenic fungi | [3] |
| T. asperellum | CuONPs | 10–190 nm | spherical | development of anticancer nanotherapeutics | [60] |
| T. harzianum | CuONPs | ~20 nm | spherical structure | Antibacterial activity | [62] |
| T. harzianum | AgNPs | 5–18 nm | spherical | Control of plant pathogens | [61] |
| CuONPs | 38–77 nm | Dispersed and elongated fibers in shape | |||
| ZnONPs | 27–40 nm in width | fan and bouquet structure | Control of microorganisms | ||
| 134–200 nm in length | |||||
| T. atroviride and 2 other fungi | CuONPs | 5–25 nm | spherical | Management of some tea plantation diseases | [63] |
| SiO2NPs | 12–22 nm | ||||
| T. asperellum | SeNPs | 49.5–312.5 nm | hexagonal, near-spherical, and irregular | Control of Sclerospora graminicola, mildew disease causative in pearl | [64] |
| T. harzianum JF309 | SeNPs | bigger than traditional SNP | irregular | antifungal | [65] |
| Trichoderma sp. WL-Go | SeNPs | An average of 147.1 nm | spherical and pseudo-spherical | ND | [66] |
| T. viride | AuNPs | 20–30 nm | spherical | bioremediation | [67] |
| T. viride | Chitosan NPs | 89.03 nm | nearly spherical | Control of soil borne pathogens | [68] |
| T. harzianum MF780864 | SiO2NPs | 89 nm | oval, rod, and cubical | Bioremediation | [69] |
| T. harzianum | iron oxide NPs | 207 ± 2 nm (α-Fe2O3) | Spherical shape | Control of S. sclerotiorum | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghuthaymi, M.A.; Abd-Elsalam, K.A.; AboDalam, H.M.; Ahmed, F.K.; Ravichandran, M.; Kalia, A.; Rai, M. Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. J. Fungi 2022, 8, 367. https://doi.org/10.3390/jof8040367
Alghuthaymi MA, Abd-Elsalam KA, AboDalam HM, Ahmed FK, Ravichandran M, Kalia A, Rai M. Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. Journal of Fungi. 2022; 8(4):367. https://doi.org/10.3390/jof8040367
Chicago/Turabian StyleAlghuthaymi, Mousa A., Kamel A. Abd-Elsalam, Hussien M. AboDalam, Farah K. Ahmed, Mythili Ravichandran, Anu Kalia, and Mahendra Rai. 2022. "Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems" Journal of Fungi 8, no. 4: 367. https://doi.org/10.3390/jof8040367
APA StyleAlghuthaymi, M. A., Abd-Elsalam, K. A., AboDalam, H. M., Ahmed, F. K., Ravichandran, M., Kalia, A., & Rai, M. (2022). Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. Journal of Fungi, 8(4), 367. https://doi.org/10.3390/jof8040367