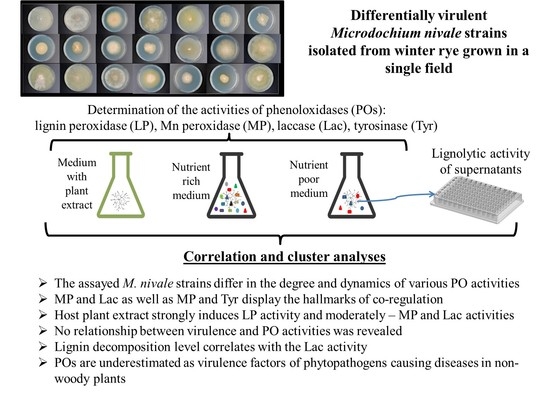
Differential Activity of the Extracellular Phenoloxidases in Different Strains of the Phytopathogenic Fungus, Microdochium nivale
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Enzymatic Activity Assays
2.3. Electrophoresis in Polyacrylamide Gel and Staining of Phenol Oxidases
2.4. Lignin Decomposition Assay
2.5. Statistical Analysis
3. Results
3.1. The Dynamics of Activities of Extracellular POs in the Cultures of Different M. nivale Strains
3.2. The Analysis of M. nivale POs by Native Gel-Eletrophoresis
3.3. The Effect of Rye Extract on the Activities of POs of M. nivale
3.4. The Comparison of PO Activities with the Virulence of M. nivale Strains
3.5. Extracellular Lignolytic Activity of M. nivale Strains
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J.G.M. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant–pathogen interactions. Front. Plant Sci. 2015, 6, 584. [Google Scholar] [CrossRef]
- Berlemont, R. Distribution and diversity of enzymes for polysaccharide degradation in fungi. Sci. Rep. 2017, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, A.R. Lignification in plant cell walls. In International Review of Cytology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 176, pp. 87–132. [Google Scholar]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Role of lignification in plant defense. Plant Signal. Behav. 2009, 4, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Slomczynski, D.; Nakas, J.P.; Tanenbaum, S.W. Production and characterization of laccase from Botrytis cinerea 61-34. Appl. Environ. Microbiol. 1995, 61, 907–912. [Google Scholar] [CrossRef]
- Lozovaya, V.V.; Lygin, A.V.; Zernova, O.V.; Li, S.; Widholm, J.M.; Hartman, G.L. Lignin degradation by Fusarium solani f. sp. glycines. Plant Dis. 2006, 90, 77–82. [Google Scholar] [CrossRef]
- Cañero, D.C.; Roncero, M.I.G. Functional analyses of laccase genes from Fusarium oxysporum. Phytopathology 2008, 98, 509–518. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Matouskova, P.; Haronikova, A.; Lichnova, A. Production of lignocellulose-degrading enzymes employing Fusarium solani F-552. Folia Microbiol. 2012, 57, 221–227. [Google Scholar] [CrossRef]
- Lin, S.Y.; Okuda, S.; Ikeda, K.; Okuno, T.; Takano, Y. LAC2 encoding a secreted laccase is involved in appressorial melanization and conidial pigmentation in Colletotrichum orbiculare. MPMI 2012, 25, 1552–1561. [Google Scholar] [CrossRef]
- Hatakka, A. Lignin-modifying enzymes from selected white-rot fungi: Production and role from in lignin degradation. FEMS Microbiol. Rev. 1994, 13, 125–135. [Google Scholar] [CrossRef]
- Hatakka, A. Biodegradation of lignin. In Biopolymers. Biology, Chemistry, Biotechnology, Applications. Vol. 1. Lignin, Humic Substances and Coal; Hofrichter, M., Steinbüchel, A., Eds.; Wiley-VCH: Weinheim, Germany, 2001; Volume 1, pp. 129–180. [Google Scholar]
- Leonowicz, A.; Matuszewska, A.; Luterek, J.; Ziegenhagen, D.; Wojtaś-Wasilewska, M.; Cho, N.-S.; Hofrichter, M.; Rogalski, J. Biodegradation of lignin by white rot fungi. Fungal Genet. Biol. 1999, 27, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Rybczyńska, K. Screening of microscopic fungi and their enzyme activities for decolorization and biotransformation of some aromatic compounds. Int. J. Environ. Sci. Technol. 2015, 12, 2673–2686. [Google Scholar] [CrossRef]
- Barapatre, A.; Jha, H. Degradation of alkali lignin by two ascomycetes and free radical scavenging activity of the products. Biocatal. Biotransform. 2017, 35, 269–286. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C.; Gil-ad, N.L. Mechanisms of survival of necrotrophic fungal plant pathogens in hosts expressing the hypersensitive response. Phytochemistry 2001, 58, 33–41. [Google Scholar] [CrossRef]
- Srivastava, P.; Andersen, P.C.; Marois, J.J.; Wright, D.L.; Srivastava, M.; Harmon, P.F. Effect of phenolic compounds on growth and ligninolytic enzyme production in Botryosphaeria isolates. Crop Prot. 2013, 43, 146–156. [Google Scholar] [CrossRef]
- Xiao, F.; Xu, W.; Hong, N.; Wang, L.; Zhang, Y.; Wang, G. A secreted lignin peroxidase required for fungal growth and virulence and related to plant immune response. Int. J. Mol. Sci. 2022, 23, 6066. [Google Scholar] [CrossRef]
- Junghanns, C.; Moeder, M.; Krauss, G.; Martin, C.; Schlosser, D. Degradation of the xenoestrogen nonylphenol by aquatic fungi and their laccases. Microbiology 2005, 151, 45–57. [Google Scholar] [CrossRef]
- Junghanns, C.; Parra, R.; Keshavarz, T.; Schlosser, D. Towards higher laccase activities produced by aquatic ascomycetous fungi through combination of elicitors and an alternative substrate. Eng. Life Sci. 2008, 8, 277–285. [Google Scholar] [CrossRef]
- Tetsch, L.; Bend, J.; Janßen, M.; Hölker, U. Evidence for functional laccases in the acidophilic ascomycete Hortaea acidophila and isolation of laccase-specific gene fragments. FEMS Microbiol. Lett. 2005, 245, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Tetsch, L.; Bend, J.; Hölker, U. Molecular and enzymatic characterisation of extra- and intracellular laccases from the acidophilic ascomycete Hortaea acidophila. Antonie Leeuwenhoek 2006, 90, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Junghanns, C.; Pecyna, M.J.; Böhm, D.; Jehmlich, N.; Martin, C.; von Bergen, M.; Schauer, F.; Hofrichter, M.; Schlosser, D. Biochemical and molecular genetic characterisation of a novel laccase produced by the aquatic ascomycete Phoma sp. UHH 5-1-03. Appl. Microbiol. Biotechnol. 2009, 84, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.; Zhang, W.; Wang, W.; Mu, Z. Isolation, identification and application in lignin degradation of an ascomycete GHJ-4. Afr. J. Biotechnol. 2011, 10, 4166–4174. [Google Scholar]
- Chefetz, B.; Chen, Y.; Hadar, Y. Purification and characterization of laccase from Chaetomium thermophilium and its role in humification. Appl. Environ. Microbiol. 1998, 64, 3175–3179. [Google Scholar] [CrossRef] [PubMed]
- Kiiskinen, L.-L.; Viikari, L.; Kruus, K. Purification and Characterisation of a novel laccase from the ascomycete Melanocarpus albomyces. Appl. Microbiol. Biotechnol. 2002, 59, 198–204. [Google Scholar] [PubMed]
- Kallio, J.P.; Auer, S.; Jänis, J.; Andberg, M.; Kruus, K.; Rouvinen, J.; Koivula, A.; Hakulinen, N. Structure–function studies of a Melanocarpus albomyces laccase suggest a pathway for oxidation of phenolic compounds J. Mol. Biol. 2009, 392, 895–909. [Google Scholar] [CrossRef]
- Hölker, U.; Schmiers, H.; Große, S.; Winkelhöfer, M.; Polsakiewicz, M.; Ludwig, S.; Dohse, J.; Höfer, M. Solubilization of low-rank coal by Trichoderma atroviride: Evidence for the involvement of hydrolytic and oxidative enzymes by using 14c-labelled lignite. JIMB 2002, 28, 207–212. [Google Scholar] [CrossRef]
- Chakroun, H.; Mechichi, T.; Martinez, M.J.; Dhouib, A.; Sayadi, S. Purification and characterization of a novel laccase from the ascomycete Trichoderma atroviride: Application on bioremediation of phenolic compounds. Process Biochem. 2010, 45, 507–513. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Savitha, S.; Swaminathan, K. Redox-Mediated Decolorization of recalcitrant textile dyes by Trichoderma harzianum WL1 laccase. World J. Microbiol. Biotechnol. 2009, 25, 1733–1741. [Google Scholar] [CrossRef]
- Gonzales, L.; Hernández, J.R.; Perestelo, F.; Carnicero, A.; Falcón, M.A. Relationship between mineralization of synthetic lignins and the generation of hydroxyl radicals by laccase and a low molecular weight substance produced by Petriellidium fusoideum. Enzyme Microb. Technol. 2002, 30, 474–481. [Google Scholar] [CrossRef]
- Edens, W.A.; Goins, T.Q.; Dooley, D.; Henson, J.M. Purification and characterization of a secreted laccase of Gaeumannomyces graminis var. tritici. Appl. Environ. Microbiol. 1999, 65, 3071–3074. [Google Scholar] [CrossRef] [Green Version]
- Thakker, G.D.; Evans, C.S.; Rao, K.K. Purification and characterization of laccase from Monocillium indicum saxena. Appl. Microbiol. Biotechnol. 1992, 37, 321–323. [Google Scholar] [CrossRef]
- Germann, U.A.; Müller, G.; Hunziker, P.E.; Lerch, K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J. Biol. Chem. 1988, 263, 885–896. [Google Scholar] [CrossRef]
- Liers, C.; Ullrich, R.; Steffen, K.T.; Hatakka, A.; Hofrichter, M. Mineralization of 14c-labelled synthetic lignin and extracellular enzyme activities of the wood-colonizing ascomycetes Xylaria hypoxylon and Xylaria polymorpha. Appl. Microbiol. Biotechnol. 2006, 69, 573–579. [Google Scholar] [CrossRef]
- Liers, C.; Ullrich, R.; Pecyna, M.; Schlosser, D.; Hofrichter, M. Production, purification and partial enzymatic and molecular characterization of a laccase from the wood-rotting ascomycete Xylaria polymorpha. Enzyme Microb. Technol. 2007, 41, 785–793. [Google Scholar] [CrossRef]
- Lopez, M.J.; del Carmen Vargas-García, M.; Suárez-Estrella, F.; Nichols, N.N.; Dien, B.S.; Moreno, J. Lignocellulose-degrading enzymes produced by the ascomycete Coniochaeta ligniaria and related species: Application for a lignocellulosic substrate treatment. Enzyme Microb. Technol. 2007, 40, 794–800. [Google Scholar] [CrossRef]
- Ferrari, R.; Gautier, V.; Silar, P. Chapter Three—Lignin Degradation by Ascomycetes. In Wood Degradation and Ligninolytic Fungi; Morel-Rouhier, M., Sormani, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 99, pp. 77–113. [Google Scholar]
- Glynn, N.C.; Hare, M.C.; Parry, D.W.; Edwards, S.G. Phylogenetic analysis of ef-1 alpha gene sequences from isolates of Microdochium nivale leads to elevation of varieties majus and nivale to species status. Mycol. Res. 2005, 109, 872–880. [Google Scholar] [CrossRef]
- Maurin, N.; Rezanoor, H.N.; Lamkadmi, Z.; Somé, A.; Nicholson, P. A Comparison of biological, molecular and enzymatic markers to investigate variability within Microdochium nivale (Fries) Samuels and Hallett. Agronomie 1995, 15, 39. [Google Scholar] [CrossRef]
- Diamond, H.; Cooke, B.M. Host specialisation in Microdochium nivale on cereals. Cereal Res. Commun. 1997, 25, 533–538. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Hsiang, T.; Yang, L. Genetic diversity of Microdochium nivale isolates from turfgrass. Mycol. Res. 1998, 102, 559–567. [Google Scholar] [CrossRef]
- Simpson, D.R.; Thomsett, M.A.; Nicholson, P. Competitive Interactions between Microdochium nivale var. majus, M. nivale var. nivale and Fusarium culmorum in planta and in vitro. Environ. Microbiol. 2004, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Ioos, R.; Belhadj, A.; Menez, M.; Faure, A. The effects of fungicides on Fusarium spp. and Microdochium nivale and their associated trichothecene mycotoxins in french naturally-infected cereal grains. Crop Prot. 2005, 24, 894–902. [Google Scholar] [CrossRef]
- Matsumoto, N. Snow Molds: A Group of Fungi That Prevail under Snow. Microbes Environ. 2009, 24, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, O.B. Snow mold fungi in Russia. In Plant and Microbe Adaptations to Cold in a Changing World; Imai, R., Yoshida, M., Matsumoto, N., Eds.; Springer: New York, NY, USA, 2013; pp. 293–303. [Google Scholar]
- Ponomareva, M.L.; Gorshkov, V.Y.; Ponomarev, S.N.; Korzun, V.; Miedaner, T. Snow mold of winter cereals: A complex disease and a challenge for resistance breeding. Theor. Appl. Genet. 2021, 134, 419–433. [Google Scholar] [CrossRef]
- Abdelhalim, M.; Brurberg, M.B.; Hofgaard, I.S.; Rognli, O.A.; Tronsmo, A.M. Pathogenicity, host specificity and genetic diversity in norwegian isolates of Microdochium nivale and Microdochium majus. Eur. J. Plant. Pathol. 2020, 156, 885–895. [Google Scholar] [CrossRef]
- Gorshkov, V.; Osipova, E.; Ponomareva, M.; Ponomarev, S.; Gogoleva, N.; Petrova, O.; Gogoleva, O.; Meshcherov, A.; Balkin, A.; Vetchinkina, E.; et al. Rye snow mold-associated Microdochium nivale strains inhabiting a common area: Variability in genetics, morphotype, extracellular enzymatic activities, and virulence. J. Fungi 2020, 6, 335. [Google Scholar] [CrossRef]
- Orth, A.B.; Royse, D.J.; Tien, M. Ubiquity of lignin-degrading peroxidases among various wood-degrading fungi. Appl. Environ. Microbiol. 1993, 59, 4017–4023. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.J.; Ruiz-Dueñas, F.J.; Guillén, F.; Martínez, A.T. Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur. J. Biochem. 1996, 237, 424–432. [Google Scholar] [CrossRef]
- Pomerantz, S.H.; Murthy, V.V. Purification and properties of tyrosinases from Vibrio tyrosinaticus. Arch. Biochem. Biophys. 1974, 160, 73–82. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Gaal, O.; Medgyesi, G.A.; Vereczkey, L. Electrophoresis in the Separation of Biological Macromolecules; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Glenn, J.K.; Gold, M.H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1985, 242, 329–341. [Google Scholar]
- Ahmad, M.; Taylor, C.R.; Pink, D.; Burton, K.; Eastwood, D.; Bending, G.D.; Bugg, T.D. Development of novel assays for lignin degradation: Comparative analysis of bacterial and fungal lignin degraders. Mol. Biosyst. 2010, 6, 815–821. [Google Scholar] [CrossRef]
- Janusz, G.; Kucharzyk, K.H.; Pawlik, A.; Staszczak, M.; Paszczynski, A.J. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzyme Microb. Technol. 2013, 52, 1–12. [Google Scholar] [PubMed]
- Tsers, I.; Meshcherov, A.; Gogoleva, O.; Petrova, O.; Gogoleva, N.; Ponomareva, M.; Gogolev, Y.; Korzun, V.; Gorshkov, V. Alterations in the transcriptome of rye plants following the Microdochium nivale infection: Identification of resistance/susceptibility-related reactions based on RNA-Seq analysis. Plants 2021, 10, 2723. [Google Scholar] [CrossRef] [PubMed]
- Tronsmo, A.M.; Hsiang, T.; Okuyama, H.; Nakajima, T. Low temperature plant microbe interactions under snow. HARC 2001, 7, 75–85. [Google Scholar]
- Matsumoto, N.; Hsiang, T. Snow Mold: The Battle under Snow between Fungal Pathogens and Their Plant Hosts; Springer: Singapore, 2016. [Google Scholar]
- Piscitelli, A.; Giardina, P.; Lettera, V.; Pezzella, C.; Sannia, G.; Faraco, V. Induction and transcriptional regulation of laccases in fungi. Curr. Genom. 2011, 12, 104–112. [Google Scholar] [CrossRef]
- Thurston, C.F.Y. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar]
- Baldrian, P. Fungal Laccases—Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar]
- Nerud, F.; Mišurcová, Z. Distribution of ligninolytic enzymes in selected white-rot fungi. Folia Microbiol. 1996, 41, 264–266. [Google Scholar]
- Steffen, K.T.; Hofrichter, M.; Hatakka, A. Mineralisation of 14c-labelled synthetic lignin and ligninolytic enzyme activities of litter-decomposing basidiomycetous fungi. Appl. Microbiol. Biotechnol. 2000, 54, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Hofrichter, M. Review: Lignin Conversion by manganese peroxidase (MnP). Enzyme Microb. Technol. 2002, 30, 454–466. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetchinkina, E.; Meshcherov, A.; Gorshkov, V. Differential Activity of the Extracellular Phenoloxidases in Different Strains of the Phytopathogenic Fungus, Microdochium nivale. J. Fungi 2022, 8, 918. https://doi.org/10.3390/jof8090918
Vetchinkina E, Meshcherov A, Gorshkov V. Differential Activity of the Extracellular Phenoloxidases in Different Strains of the Phytopathogenic Fungus, Microdochium nivale. Journal of Fungi. 2022; 8(9):918. https://doi.org/10.3390/jof8090918
Chicago/Turabian StyleVetchinkina, Elena, Azat Meshcherov, and Vladimir Gorshkov. 2022. "Differential Activity of the Extracellular Phenoloxidases in Different Strains of the Phytopathogenic Fungus, Microdochium nivale" Journal of Fungi 8, no. 9: 918. https://doi.org/10.3390/jof8090918
APA StyleVetchinkina, E., Meshcherov, A., & Gorshkov, V. (2022). Differential Activity of the Extracellular Phenoloxidases in Different Strains of the Phytopathogenic Fungus, Microdochium nivale. Journal of Fungi, 8(9), 918. https://doi.org/10.3390/jof8090918