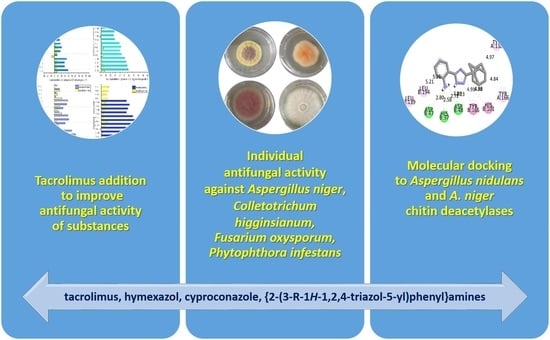
Combined Application of Tacrolimus with Cyproconazole, Hymexazol and Novel {2-(3-R-1H-1,2,4-triazol-5-yl)phenyl}amines as Antifungals: In Vitro Growth Inhibition and In Silico Molecular Docking Analysis to Fungal Chitin Deacetylase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antifungal Studies
2.2. Molecular Docking Studies
3. Results and Discussion
3.1. Antifungal Studies
3.2. Molecular Docking
4. Proposed Activity Mechanisms and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security—Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar] [CrossRef]
- Leisner, C.L. Climate change impacts on food security- focus on perennial cropping systems and nutritional value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef] [PubMed]
- von Braun, J. Bioeconomy—The global trend and its implications for sustainability and food security. Glob. Food Secur. 2018, 19, 81–83. [Google Scholar] [CrossRef]
- Brück, T.; d’Errico, M. Food security and violent conflict: Introduction to the special issue. World Dev. 2019, 119, 145–149. [Google Scholar] [CrossRef]
- Majeed, A. Application of agrochemicals in agriculture: Benefits, risks and responsibility of stakeholders. J. Food Sci. Toxicol. 2018, 2, 1–2. [Google Scholar]
- Andrade, C.; Villers, A.; Balent, G.; Bar-Hen, A.; Chadoeuf, J.; Cylly, D.; Cluzeau, D.; Fried, G.; Guillocheau, S.; Pillon, O.; et al. A real-world implementation of a nationwide, long-term monitoring program to assess the impact of agrochemicals and agricultural practices on biodiversity. Ecol. Evol. 2021, 11, 3771–3793. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, P.A.; Abubakar, S.K.; Adesiji, R.A. Effect of agrochemicals on groundwater quality: A Review. Sci. Agri. 2013, 1, 1–7. [Google Scholar]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Brauer, V.S.; Rezende, C.P.; Pessoni, A.M.; De Paula, R.G.; Rangappa, K.S.; Nayaka, S.C.; Gupta, V.K.; Almeida, F. Antifungal Agents in Agriculture: Friends and Foes of Public Health. Biomolecules 2019, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, N.J.; Fraaije, B.A. Fitness Penalties in the Evolution of Fungicide Resistance. Annu. Rev. Phytopathol. 2018, 56, 339–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Bosch, F.; Oliver, R.; van den Berg, F.; Paveley, N. Governing Principles Can Guide Fungicide-Resistance Management Tactics. Annu. Rev. Phytopathol. 2014, 52, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Antypenko, L.; Sadykova, Z.; Shabelnyk, K.; Meyer, F.; Kovalenko, S.; Meyer, V.; Garbe, L.A.; Steffens, K. Synthesis and mode of action studies of novel {2-(3-R-1H-1,2,4-triazol-5-yl)phenyl}amines to combat pathogenic fungi. Arch. Pharm. 2019, 352, e1900092. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.N.; Heick, T.M. Azole Use in Agriculture, Horticulture, and Wood Preservation—Is It Indispensable? Front. Cell. Infect. Microbiol. 2021, 11, 730297. [Google Scholar] [CrossRef]
- Riise, G.; Lundekvam, H.; Wu, Q.L.; Haugen, L.E.; Mulder, J. Loss of pesticides from agricultural fields in SE Norway–runoff through surface and drainage water. Environ. Geochem. Health 2004, 26, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef] [Green Version]
- Kathiravana, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 678–5698. [Google Scholar] [CrossRef]
- PPDB: Pesticide Properties DataBase. Cyproconazole. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/198.htm#:~:text=Based%20on%20its%20physico%2Dchemical,aquatic%20organisms%2C%20earthworms%20and%20honeybees (accessed on 22 April 2022).
- Lewis, K.A.; Tzilivakis, J.; Warner, D.; Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016, 22, 1050–1064. [Google Scholar] [CrossRef] [Green Version]
- PPDB: Pesticide Properties DataBase. Hymexazol. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/Reports/388.htm (accessed on 22 April 2022).
- Sun, D.; Li, L.; Ji, R.; Li, W.; Ye, H.C.; Wu, Y.J.; Liu, C.L. Determination of Hymexazol in Cucumber and Soil Samples by Derivatization Using GC-FPD. Bull. Environ. Contam. Toxicol. 2011, 87, 653–656. [Google Scholar] [CrossRef]
- Vera, T.; Muñoz, A.; Ródenas, M.; Vázquez, M.; Borrás, E.; Marqués, M.; Mellouki, A.; Treacy, J.; Sidebottom, H. Atmospheric fate of hymexazol (5-methylisoxazol-3-ol): Simulation chamber studies. Atmos. Environ. 2011, 45, 3704–3710. [Google Scholar] [CrossRef]
- Ma, X.-P.; Chen, J.-S.; Du, X.-H. A continuous flow process for the synthesis of hymexazol. Org. Process Res. Dev. 2019, 23, 1152–1158. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Xue, M.; Liu, Z.; Zhang, Q.; Hou, J.; Xing, M.; Wang, R.; Liu, T. The combination of a biocontrol agent Trichoderma asperellum SC012 and hymexazol reduces the effective fungicide dose to control Fusarium wilt in cowpea. J. Fungi 2021, 7, 685. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, W.; Jia, L.; Li, L.; Zhao, J.; Zhao, Z.; Peng, S.; Chen, Y.; Yuan, X. Combined Developmental Toxicity of the Pesticides Difenoconazole and Dimethomorph on Embryonic Zebrafish. Toxins 2021, 13, 854. [Google Scholar] [CrossRef] [PubMed]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Evaluation of plant-growth-promoting rhizobacteria, acibenzolar-S-methyl and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest Manag. Sci. 2012, 68, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Antypenko, L.; Meyer, F.; Sadykova, Z.; Garbe, L.-A.; Steffens, K.G. Tacrolimus as Antifungal Agent. Acta Chim. Slov. 2019, 66, 784–791. [Google Scholar] [CrossRef]
- Tanaka, H.; Kuroda, A.; Marusawa, H.; Hatanaka, H.; Kino, T.; Goto, T.; Hashimoto, M.; Taga, T. Structure of FK506, a novel immunosuppressant isolated from Streptomyces. J. Am. Chem. Soc. 1987, 109, 5031–5033. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Reedy, J.L.; Cramer, R.A., Jr.; Perfect, J.R.; Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007, 5, 418–430. [Google Scholar] [CrossRef]
- Sanglard, D.; Ischer, F.; Marchetti, O.; Entenza, J.; Bille, J. Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 2003, 48, 959–976. [Google Scholar] [CrossRef]
- Liu, S.; Hou, Y.; Liu, W.; Lu, C.; Wang, W.; Sun, S. Components of the calcium-calcineurin signaling pathway in fungal cells and their potential as antifungal targets. Eukaryot. Cell 2015, 14, 324–334. [Google Scholar] [CrossRef] [Green Version]
- PPDB: Pesticide Properties DataBase. Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 23 April 2022).
- Cui, J.; Ren, B.; Tong, Y.; Dai, H.; Zhang, L. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence 2015, 6, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Ren, B.; Chen, M.; Liu, M.X.; Ren, W.; Wang, Q.X.; Zhang, L.X.; Yan, G.Y. ASDCD: Antifungal synergistic drug combination database. PLoS ONE 2014, 9, e86499. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Liu, L.; Li, L.; Tan, L.; Sun, Y. The synergistic effect of tacrolimus (FK506) or everolimus and azoles against Scedosporium and Lomentospora species in vivo and in vitro. Front. Cell. Infect. Microbiol. 2022, 12, 864912. [Google Scholar] [CrossRef] [PubMed]
- Bonin, M.; Hameleers, L.; Hembach, L.; Roret, T.; Cord-Landwehr, S.; Michel, G.; Moerschbacher, B.M. In silico and in vitro analysis of an Aspergillus niger chitin deacetylase to decipher its subsite sugar preferences. J. Biol. Chem. 2021, 297, 101129. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.G.; Specht, C.A.; Donlin, M.J.; Lodge, J.K. Chitosan, the deacetylated form of chitin, is necessary for cell wall integrity in Cryptococcus neoformans. Eukaryot. Cell 2007, 6, 855–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BRENDA:EC2.4.1.16 Information on EC 2.4.1.16—Chitin Synthase. Available online: https://www.brenda-enzymes.info/enzyme.php?ecno=2.4.1.16 (accessed on 1 July 2022).
- Liu, Y.; Ahmed, S.; Fang, Y.; Chen, M.; An, J.; Yang, G.; Hou, X.; Lu, J.; Ye, Q.; Zhu, R.; et al. Discovery of chitin deacetylase inhibitors through structure-based virtual screening and biological assays. J. Microbiol. Biotechnol. 2022, 32, 504–513. [Google Scholar] [CrossRef]
- Endo, A.; Kakiki, K.; Misato, T. Mechanism of action of the antifugal agent Polyoxin D. J. Bacteriol. 1970, 104, 189–196. [Google Scholar] [CrossRef] [Green Version]
- Polyoxin, D. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Polyoxin-d (accessed on 1 May 2022).
- Protein Data Bank. Structure of the Chitin Deacetylase AngCDA from Aspergillus niger. 7BLY. Available online: https://www.rcsb.org/structure/7BLY (accessed on 24 April 2022).
- Protein Data Base. A. nidulans chitin deacetylase. 2Y8U. Available online: https://www.rcsb.org/structure/2Y8U (accessed on 1 May 2022).
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Baber, J.C.; Thompson, D.C.; Cross, J.B.; Humblet, C. GARD: A generally applicable replacement for RMSD. J. Chem. Inf. Model. 2009, 49, 1889–1900. [Google Scholar] [CrossRef]
- Warren, G.L.; Andrews, C.W.; Capelli, A.-M.; Clarke, B.; LaLonde, J.; Lambert, M.H.; Lindvall, M.; Nevins, N.; Semus, S.F.; Senger, S.; et al. A Critical Assessment of Docking Programs and Scoring Functions. J. Med. Chem. 2006, 49, 5912–5931. [Google Scholar] [CrossRef]
- DockRMSD. Docking Pose Distance Calculation. Available online: https://seq2fun.dcmb.med.umich.edu//DockRMSD (accessed on 1 July 2022).
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.-R.; Shilts, T.; Li, W.; Timmer, L. Engineering a genetic transformation system for Colletotrichum acutatum, the causal fungus of lime anthracnose and postbloom fruit drop of citrus. FEMS Microbiol. Lett. 2002, 213, 33–39. [Google Scholar] [CrossRef]
- Ruiz, A.; Parra, C.C.; da Graca, J.V.; Salas, B.; Malik, N.S.A.; Kunta, M. Molecular characterization and pathogenicity assays of Colletotrichum acutatum, causal agent for lime anthracnose in Texas. Rev. Mex. Fitopatol. 2014, 32, 52–61. [Google Scholar]
- Rojo-Báez, I.; Álvarez-Rodríguez, B.; García-Estrada, R.; León-Félix, J.; Sañudo-Barajas, A.; Allende-Molar, R. Current status of Colletotrichum spp. in Mexico: Taxonomy, characterization, pathogenesis and control. Rev. Mex. Fitopal. 2017, 35, 549–570. [Google Scholar]
- Cruz-Lagunas, B.; Ortega-Acosta, S.Á.; Reyes-García, G.; Toribio-Jiménez, J.; Juárez-López, P.; Guillén-Sánchez, D.; Damián-Nava, A.; Romero-Ramírez, Y.; Palemón-Alberto, F. Colletotrichum gloeosporioides causes anthracnose on grapefruit (Citrus paradisi) in Mexico Australas. Plant Dis. Notes 2020, 15, 31. [Google Scholar] [CrossRef]
- Xavier, K.V.; Achala, N.K.C.; Peres, N.A.; Deng, Z.; Castle, W.; Lovett, W.; Vallad, G.E. Characterization of Colletotrichum species causing anthracnose of pomegranate in the southeastern United States. Plant Dis. 2019, 103, 2771–2780. [Google Scholar] [CrossRef]
- D’Mello, J.P.F.; Macdonald, A.M.C.; Postel, D.; Dijksma, W.T.P.; Dujardin, A.; Placinta, C.M. Pesticide Use and Mycotoxin Production in Fusarium and Aspergillus Phytopathogens. Eur. J. Plant Pathol. 1998, 104, 741–751. [Google Scholar] [CrossRef]
- Farquhar, M.L.; Peterson, R.L. Induction of protoplast formation in the ectomycorrhizal fungus Paxillus involutus by the root rot pathogen Fusarium oxysporum. New Phytol. 2010, 116, 107–113. [Google Scholar] [CrossRef]
- Gordon, T.R. Fusarium oxysporum and the Fusarium Wilt Syndrome. Annu. Rev. Phytopathol. 2017, 55, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Galvez, C.; Champouret, N.; Rietman, H.; Lin, X.; Wouters, D.; Chu, Z.; Jones, J.D.G.; Vossen, J.H.; Visser, R.G.F.; Wolters, P.J.; et al. Two different R gene loci co-evolved with Avr2 of Phytophthora infestans and confer distinct resistance specificities in potato. Stud. Mycol. 2018, 89, 105–115. [Google Scholar] [CrossRef]
- Mazumdar, P.; Singh, P.; Kethiravan, D.; Ramathani, I.; Ramakrishnan, N. Late blight in tomato: Insights into the pathogenesis of the aggressive pathogen Phytophthora infestans and future research priorities. Planta 2021, 253, 119. [Google Scholar] [CrossRef] [PubMed]
- Dannaoui, E.; Schwarz, P.; Lortholary, O. In vitro interactions between antifungals and immunosuppressive drugs against zygomycetes. Antimicrob. Agents Chemother. 2009, 53, 3549–3551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stebbing, A.R.D. A mechanism for hormesis—A problem in the wrong discipline. Crit. Rev. Toxicol. 2003, 33, 463–467. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Baldwin, L.A. Toxicology rethinks its central belief—Hormesis demands a reappraisal of the way risks are assessed. Nature 2003, 421, 691–692. [Google Scholar] [CrossRef]
- Rösch, A.; Gottardi, M.; Vignet, C.; Cedergreen, N.; Hollender, J. Mechanistic understanding of the synergistic potential of azole fungicides in the aquaticin vertebrate Gammarus pulex. Environ. Sci. Technol. 2017, 51, 12784–12795. [Google Scholar] [CrossRef] [PubMed]
- Antypenko, L.; Shabelnyk, K.; Kovalenko, S. Tacrolimus and azole derivatives of agricultural and human health importance: Prediction of ADME properties. Curr. Comput. Aided Drug Des. 2022. under review. [Google Scholar]
- Zanni, R.; Galvez-Llompart, M.; García-Domenech, R.; Galvez, J. What place does molecular topology have in today’s drug discovery? Expert. Opin. Drug Discov. 2020, 15, 1133–1144. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.M.; Polonio, Á.; Zanni, R.; Romero, D.; Gálvez, J.; Fernández-Ortuño, D.; Pérez-García, A. Chitin deacetylase, a novel target for the design of agricultural fungicides. J. Fungi 2021, 7, 1009. [Google Scholar] [CrossRef]
- Pacheco, N.; Trombotto, S.; David, L.; Shirai, K. Activity of chitin deacetylase from Colletotrichum gloeosporioides on chitinous substrates. Carbohydr. Polym. 2013, 96, 227–232. [Google Scholar] [CrossRef]
- Takeguchi, N.; Ichimura, K.; Koike, M.; Matsui, W.; Kashiwagura, T.; Kawahara, K. Inhibition of the multidrug efflux pump in isolated hepatocyte couplets by immunosuppressants FK506 and cyclosporine. Transplantation 1993, 55, 646–650. [Google Scholar] [CrossRef]
- Saad, A.H.; De Pestel, D.D.; Carver, P.L. Factors influencing the magnitude and clinical significance of drug interactions between azole antifungals and select immunosuppressants. Pharmacotherapy 2006, 26, 1730–1744. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Ji, D.; Zhang, Z.; Li, B.; Qin, G.; Xu, Y.; Chen, T.; Tian, S. Efficacy of rapamycin in modulating autophagic activity of Botrytis cinerea for controlling gray mold. Postharvest Biol. Technol. 2019, 150, 158–165. [Google Scholar] [CrossRef]
- De Waard, M.A. Synergism and antagonism in fungicide mixtures containing sterol demethylation inhibitor. Phytopathology 1996, 86, 1280–1283. [Google Scholar]
- Thomas, P.P.; Manivannan, J.; John, G.T.; Jacob, C.K. Sirolimus and ketoconazole co-prescription in renal transplant recipients. Transplantation 2004, 77, 474–475. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Kakkar, S. Topical delivery of antifungal agents. Expert Opin. Drug. Deliv. 2010, 7, 1303–1327. [Google Scholar] [CrossRef]
- Güngör, S.; Erdal, M.S. Nanocarriers of Antifungal Agents. In Recent Trends in Antifungal Agents and Antifungal Therapy; Basak, A., Chakraborty, R., Mandal, S., Eds.; Springer: New Delhi, India, 2016; pp. 175–190. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Sheng, X.; Li, Q.N. Adsorption and release kinetics, equilibrium, and thermodynamic studies of hymexazol onto diatomite ping. ACS Omega 2020, 5, 29504–29512. [Google Scholar] [CrossRef]





| Substance | 7BLY | 2Y8U |
|---|---|---|
| Triazole (5) | −8.2 | −9.6 |
| J075-4187 (7) | −7.5 | −9.0 |
| Triazole (4) | −7.5 | −7.8 |
| Cyproconazole (3) | −6.5 | −7.1 |
| Polyoxorin D (6) | −6.2 | −7.1 |
| Tacrolimus (1) | −4.6 | −6.9 |
| Hymexazol (2) | −5.2 | −5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antypenko, L.; Meyer, F.; Sadyk, Z.; Shabelnyk, K.; Kovalenko, S.; Steffens, K.G.; Garbe, L.-A. Combined Application of Tacrolimus with Cyproconazole, Hymexazol and Novel {2-(3-R-1H-1,2,4-triazol-5-yl)phenyl}amines as Antifungals: In Vitro Growth Inhibition and In Silico Molecular Docking Analysis to Fungal Chitin Deacetylase. J. Fungi 2023, 9, 79. https://doi.org/10.3390/jof9010079
Antypenko L, Meyer F, Sadyk Z, Shabelnyk K, Kovalenko S, Steffens KG, Garbe L-A. Combined Application of Tacrolimus with Cyproconazole, Hymexazol and Novel {2-(3-R-1H-1,2,4-triazol-5-yl)phenyl}amines as Antifungals: In Vitro Growth Inhibition and In Silico Molecular Docking Analysis to Fungal Chitin Deacetylase. Journal of Fungi. 2023; 9(1):79. https://doi.org/10.3390/jof9010079
Chicago/Turabian StyleAntypenko, Lyudmyla, Fatuma Meyer, Zhanar Sadyk, Konstyantyn Shabelnyk, Sergiy Kovalenko, Karl Gustav Steffens, and Leif-Alexander Garbe. 2023. "Combined Application of Tacrolimus with Cyproconazole, Hymexazol and Novel {2-(3-R-1H-1,2,4-triazol-5-yl)phenyl}amines as Antifungals: In Vitro Growth Inhibition and In Silico Molecular Docking Analysis to Fungal Chitin Deacetylase" Journal of Fungi 9, no. 1: 79. https://doi.org/10.3390/jof9010079
APA StyleAntypenko, L., Meyer, F., Sadyk, Z., Shabelnyk, K., Kovalenko, S., Steffens, K. G., & Garbe, L. -A. (2023). Combined Application of Tacrolimus with Cyproconazole, Hymexazol and Novel {2-(3-R-1H-1,2,4-triazol-5-yl)phenyl}amines as Antifungals: In Vitro Growth Inhibition and In Silico Molecular Docking Analysis to Fungal Chitin Deacetylase. Journal of Fungi, 9(1), 79. https://doi.org/10.3390/jof9010079