Biphasic Porous Bijel-Like Structures with Hydrogel Domains as Controlled Drug Delivery Systems
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biphasic Porous Structure Synthesis and Characterisation
2.2. Rheological Measurements
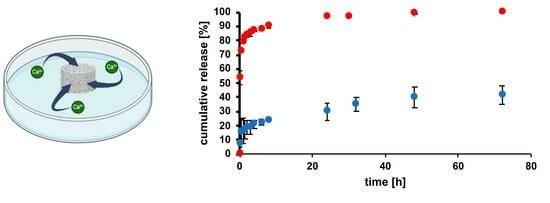
2.3. Release Tests
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Hydroxyapatite Nanoparticles Synthesis
4.3. Bijel-like Structure Synthesis
4.4. Scanning Electron Microscopy (SEM)
4.5. Differential Scanning Calorimetry (DSC)
4.6. Rheological Measurements
4.7. Swelling Tests
4.8. Release Tests
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herzig, E.M.; White, K.A.; Schofield, A.B.; Poon, W.C.K.; Clegg, P.S. Bicontinuous emulsions stabilized solely by colloidal particles. Nat. Mater. 2007, 6, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Stratford, K.; Adhikari, R.; Pagonabarraga, I.; Desplat, J.-C.; Cates, M.E. Colloidal jamming at interfaces: A route to fluid-bicontinuous gels. Science 2005, 309, 2198–2201. [Google Scholar] [CrossRef] [PubMed]
- Cates, M.E.; Clegg, P.S. Bijels: A new class of soft materials. Soft Matter 2008, 4, 2132–2138. [Google Scholar] [CrossRef]
- Wang, T.; Riggleman, R.A.; Lee, D.; Stebe, K.J. Bicontinuous interfacially jammed emulsion gels with nearly uniform sub-micrometer domains via regulated co-solvent removal. Mater. Horiz. 2023, 10, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Ching, H.; Mohraz, A. Bijel rheology reveals a 2D colloidal glass wrapped in 3D. Soft Matter 2022, 18, 4227–4238. [Google Scholar] [CrossRef] [PubMed]
- Vanoli, V.; Massobrio, G.; Pizzetti, F.; Mele, A.; Rossi, F.; Castiglione, F. Bijels as a Fluid Labyrinth for Drugs: The Effect of Nanoparticles on the Release Kinetics of Ethosuximide and Dimethyl Fumarate. ACS Omega 2022, 7, 42845–42853. [Google Scholar] [CrossRef] [PubMed]
- Di Vitantonio, G.; Wang, T.; Haase, M.F.; Stebe, K.J.; Lee, D. Robust Bijels for Reactive Separation via Silica-Reinforced Nanoparticle Layers. ACS Nano 2019, 13, 26–31. [Google Scholar] [CrossRef]
- Firoozmand, H.; Rousseau, D. Food-grade bijels based on gelatin-maltodextrin-microbial cell composites. Food Hydrocoll. 2015, 48, 208–212. [Google Scholar] [CrossRef]
- Boakye-Ansah, S.; Khan, M.A.; Haase, M.F. Controlling Surfactant Adsorption on Highly Charged Nanoparticles to Stabilize Bijels. J. Phys. Chem. C 2020, 124, 12417–12423. [Google Scholar] [CrossRef]
- Tavacoli, J.W.; Thijssen, J.H.J.; Schofield, A.B.; Clegg, P.S. Novel, robust, and versatile bijels of nitromethane, ethanediol, and colloidal silica: Capsules, sub-ten-micrometer domains, and mechanical properties. Adv. Funct. Mater. 2011, 21, 2020–2027. [Google Scholar] [CrossRef]
- Thorson, T.J.; Gurlin, R.E.; Botvinick, E.L.; Mohraz, A. Bijel-templated implantable biomaterials for enhancing tissue integration and vascularization. Acta Biomater. 2019, 94, 173–182. [Google Scholar] [CrossRef]
- McDevitt, K.M.; Thorson, T.J.; Botvinick, E.L.; Mumm, D.R.; Mohraz, A. Microstructural characteristics of bijel-templated porous materials. Materialia 2019, 7, 100393. [Google Scholar] [CrossRef]
- Kharal, S.P.; Haase, M.F. Centrifugal Assembly of Helical Bijel Fibers for pH Responsive Composite Hydrogels. Small 2022, 18, 1–9. [Google Scholar] [CrossRef]
- Thorson, T.J.; Botvinick, E.L.; Mohraz, A. Composite Bijel-Templated Hydrogels for Cell Delivery. ACS Biomater. Sci. Eng. 2018, 4, 587–594. [Google Scholar] [CrossRef]
- Pizzetti, F.; Rossetti, A.; Marchetti, A.; Castiglione, F.; Vanoli, V.; Coste, E.; Veneruso, V.; Veglianese, P.; Sacchetti, A.; Cingolani, A.; et al. Biphasic Porous Structures formed by Monomer/Water Interface Stabilization with Colloidal Nanoparticles. Adv. Mater. Interfaces 2021, 8, 2100991. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Bhaskar, R.; Han, S.S. Recent Advances in the Biomedical Applications of Functionalized Nanogels. Pharmaceutics 2022, 14, 2832. [Google Scholar] [CrossRef]
- Nassar, N.; Kasapis, S. Fundamental advances in hydrogels for the development of the next generation of smart delivery systems as biopharmaceuticals. Int. J. Pharm. 2023, 633, 122634. [Google Scholar] [CrossRef]
- Kharkar, P.M.; Kiick, K.L.; Kloxin, A.M. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem. Soc. Rev. 2013, 42, 7335–7372. [Google Scholar] [CrossRef]
- Vismara, I.; Papa, S.; Rossi, F.; Forloni, G.; Veglianese, P. Current Options for Cell Therapy in Spinal Cord Injury. Trends Mol. Med. 2017, 23, 831–849. [Google Scholar] [CrossRef]
- Jansen, F.; Harting, J. From bijels to Pickering emulsions: A lattice Boltzmann study. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2011, 83, 046707. [Google Scholar] [CrossRef]
- Reeves, M.; Brown, A.T.; Schofield, A.B.; Cates, M.E.; Thijssen, J.H.J. Particle-size effects in the formation of bicontinuous Pickering emulsions. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2015, 92, 032308. [Google Scholar] [CrossRef]
- White, K.A.; Schofield, A.B.; Binks, B.P.; Clegg, P.S. Influence of particle composition and thermal cycling on bijel formation. J. Phys. Condens. Matter 2008, 20, 494223. [Google Scholar] [CrossRef]
- Partenope, A.; Pizzetti, F.; Vanoli, V.; Casalegno, M.; Cingolani, A.; Nogueira, L.P.; Castiglione, F.; Haugen, H.J.; Rossi, F. A facile surfactant-free strategy to construct porous structures with hydrophobic and hydrophilic domains from polymer/water mixtures. Mater. Today Commun. 2022, 33, 104290. [Google Scholar] [CrossRef]
- Bai, L.; Fruehwirth, J.W.; Cheng, X.; MaCosko, C.W. Dynamics and rheology of nonpolar bijels. Soft Matter 2015, 11, 5282–5293. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Łętocha, A.; Miastkowska, M.; Sikora, E. Preparation and Characteristics of Alginate Microparticles for Food, Pharmaceutical and Cosmetic Applications. Polymers 2022, 14, 3834. [Google Scholar] [CrossRef]
- Nezamdoost-Sani, N.; Khaledabad, M.A.; Amiri, S.; Mousavi Khaneghah, A. Alginate and derivatives hydrogels in encapsulation of probiotic bacteria: An updated review. Food Biosci. 2023, 52, 102433. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Yao, Z.; Zhu, B. Recent advances in the production, properties and applications of alginate oligosaccharides—A mini review. World J. Microbiol. Biotechnol. 2023, 39, 207. [Google Scholar] [CrossRef]
- Takatsuka, S.; Kubota, T.; Kurashina, Y.; Onoe, H. Near-Infrared-Triggered On-Demand Controlled Release of Adeno-Associated Virus from Alginate Hydrogel Microbeads with Heat Transducer for Gene Therapy. Small 2023, 19, 202204139. [Google Scholar] [CrossRef]
- Harper, B.A.; Barbut, S.; Lim, L.T.; Marcone, M.F. Effect of Various Gelling Cations on the Physical Properties of “Wet” Alginate Films. J. Food Sci. 2014, 79, 562–567. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pizzetti, F.; Massobrio, G.; Riva, S.; Vangosa, F.B.; Rossi, F. Biphasic Porous Bijel-Like Structures with Hydrogel Domains as Controlled Drug Delivery Systems. Gels 2024, 10, 72. https://doi.org/10.3390/gels10010072
Pizzetti F, Massobrio G, Riva S, Vangosa FB, Rossi F. Biphasic Porous Bijel-Like Structures with Hydrogel Domains as Controlled Drug Delivery Systems. Gels. 2024; 10(1):72. https://doi.org/10.3390/gels10010072
Chicago/Turabian StylePizzetti, Fabio, Giovanna Massobrio, Silvia Riva, Francesco Briatico Vangosa, and Filippo Rossi. 2024. "Biphasic Porous Bijel-Like Structures with Hydrogel Domains as Controlled Drug Delivery Systems" Gels 10, no. 1: 72. https://doi.org/10.3390/gels10010072
APA StylePizzetti, F., Massobrio, G., Riva, S., Vangosa, F. B., & Rossi, F. (2024). Biphasic Porous Bijel-Like Structures with Hydrogel Domains as Controlled Drug Delivery Systems. Gels, 10(1), 72. https://doi.org/10.3390/gels10010072