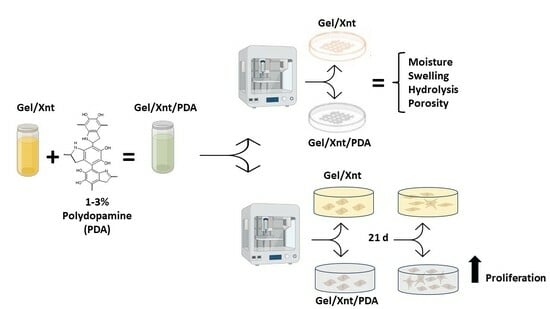
Polydopamine Blending Increases Human Cell Proliferation in Gelatin–Xanthan Gum 3D-Printed Hydrogel
Abstract
:1. Introduction
2. Results and Discussion
2.1. PDA Does Not Alter Hydrogel Printability and Moisture
2.2. PDA Blending Effect on Hydrogel Swelling
2.3. PDA Effect on Hydrogel Hydrolysis
2.4. PDA Effect on Hydrogel Porosity
2.5. PDA Effect on Cell Proliferation in Cell-Laden Hydrogel
3. Conclusions
4. Materials and Methods
4.1. Preparation of Polydopamine
4.2. Preparation of Hydrogel
4.3. Hydrogel Bioprinting and Crosslinking
4.4. Characterization of 3D-Printed Hydrogel
4.4.1. Moisture
4.4.2. Swelling
4.4.3. Hydrolysis
4.4.4. Porosity
4.5. Cell Culture
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.; Yin, D.; Li, W.; Tang, Q.; Zou, L.; Peng, Q. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf. B Biointerfaces 2021, 199, 111502. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yu, J. Mussel-Inspired Biomaterials for Cell and Tissue Engineering. Adv. Exp. Med. Biol. 2018, 1077, 451–474. [Google Scholar] [PubMed]
- Sarkari, S.; Khajehmohammadi, M.; Davari, N.; Li, D.; Yu, B. The effects of process parameters on polydopamine coatings employed in tissue engineering applications. Front. Bioeng. Biotechnol. 2022, 10, 1005413. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, K.M.; Sikorski, P. Patterned cell arrays and patterned co-cultures on polydopamine-modified poly(vinyl alcohol) hydrogels. Biofabrication 2013, 5, 045009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, B.; Zhou, Y.; Zhou, F.; Liu, W.; Wang, Z. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef]
- Hu, Y.; Dan, W.; Xiong, S.; Kang, Y.; Dhinakar, A.; Wu, J.; Gu, Z. Development of collagen/polydopamine complexed matrix as mechanically enhanced and highly biocompatible semi-natural tissue engineering scaffold. Acta Biomater. 2017, 47, 135–148. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Zare, M.; Khodadadi, A.; Seidi, F.; Sajadi, S.M.; Zarrintaj, P.; Arefi, A.; Saeb, M.R.; Mozafari, M. Polydopamine Biomaterials for Skin Regeneration. ACS Biomater. Sci. Eng. 2022, 13, 2196–2219. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Petralito, S.; Subham, S.; Gilmore, D.; Varani, G.; Yang, G.; Lin, D.; Casadei, M.A.; Paul, A. Investigating the role of polydopamine to modulate stem cell adhesion and proliferation on gellan gum-based hydrogels. ACS Appl. Bio Mater. 2020, 3, 945–951. [Google Scholar] [CrossRef]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine modified Superparamagnetic Iron oxide nanoparticles as multifunctional nanocarrier for targeted prostate cancer treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, Y.; Zuo, C.; Ao, H.; Guo, Y.; Wang, X.; Han, M. Targeted antitumor comparison study between dopamine self-polymerization and traditional synthesis for nanoparticle surface modification in drug delivery. Nanotechnology 2021, 32, 30. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Transl. 2019, 17, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic hydrogels to promote wound healing. Front. Bioeng. Biotechnol. 2021, 9, 718377. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Zhu, X.; Huang, H.; Xu, C. Performance of polydopamine complex and mechanisms in wound healing. Int. J. Mol. Sci. 2021, 22, 10563. [Google Scholar] [CrossRef]
- Zhang, S.; Ge, G.; Qin, Y.; Li, W.; Dong, J.; Mei, J.; Ma, R.; Zhang, X.; Bai, J.; Zhu, C.; et al. Recent advances in responsive hydrogels for diabetic wound healing. Mater. Today Bio 2022, 18, 100508. [Google Scholar] [CrossRef]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 989729. [Google Scholar] [CrossRef]
- Ghorai, S.K.; Dutta, A.; Roy, T.; Guha Ray, P.; Ganguly, D.; Ashokkumar, M.; Dhara, S.; Chattopadhyay, S. Metal ion augmented mussel inspired polydopamine immobilized 3D printed osteoconductive scaffolds for accelerated bone tissue regeneration. ACS Appl. Mater. Interfaces 2022, 14, 28455–28475. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yi, P.; Liu, Z.; Zhang, W.; Mei, L.; Feng, C.; Tu, C.; Li, Z. Stem cell-laden hydrogel-based 3D bioprinting for bone and cartilage tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 865770. [Google Scholar] [CrossRef] [PubMed]
- Teixeir, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A guide to polysaccharide-based hydrogel bioinks for 3D bioprinting applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef]
- Gao, Q.; Kim, B.S.; Gao, G. Advanced strategies for 3D bioprinting of tissue and organ analogs using alginate hydrogel bioinks. Mar. Drugs 2021, 19, 708. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Syed, A.; Wong, M.; Hicks, J.; Nunez, G.; Jitianu, A.; Siler, Z.; Peterson, M. Polydopamine antioxidant hydrogels for wound healing applications. Gels 2020, 6, 39. [Google Scholar] [CrossRef]
- Im, S.; Choe, G.; Seok, J.M.; Yeo, S.J.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. An osteogenic bioink composed of alginate, cellulose nanofibrils, and polydopamine nanoparticles for 3D bioprinting and bone tissue engineering. Int. J. Biol. Macromol. 2022, 205, 520–529. [Google Scholar] [CrossRef]
- Chen, Y.W.; Fang, H.Y.; Shie, M.Y.; Shen, Y.F. The mussel-inspired assisted apatite mineralized on PolyJet material for artificial bone scaffold. Int. J. Bioprint 2019, 5, 197. [Google Scholar] [CrossRef]
- Piola, B.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of gelatin-xanthan gum composite hydrogels for growth of human skin cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef] [PubMed]
- Liebscher, J.; Mrówczyński, R.; Scheidt, H.A.; Filip, C.; Hădade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Nilforoushzadeh, M.A.; Sisakht, M.M.; Amirkhani, M.A.; Seifalian, A.M.; Banafshe, H.R.; Verdi, J.; Nouradini, M. Engineered skin graft with stromal vascular fraction cells encapsulated in fibrin–collagen hydrogel: A clinical study for diabetic wound healing. J. Tissue Eng. Regen. Med. 2020, 14, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D bioprinting of novel biocompatible scaffolds for endothelial cell repair. Polymers 2019, 11, 1924. [Google Scholar] [CrossRef]
- Shawan, M.M.A.K.; Islam, N.; Aziz, S.; Khatun, N.; Sarker, S.R.; Hossain, M.; Hossan, T.; Morshed, M.; Sarkar, M.; Shakil, M.S. Fabrication of xanthan gum: Gelatin (Xnt: Gel) hybrid composite hydrogels for evaluating skin wound healing efficacy. Mod. Appl. Sci. 2019, 13, 101. [Google Scholar] [CrossRef]
- Sanbhal, N.; Saitaer, X.; Peerzada, M.; Habboush, A.; Wang, F.; Wang, L. One-step surface functionalized hydrophilic polypro-pylene meshes for hernia repair using bio-inspired polydopamine. Fibers 2019, 7, 6. [Google Scholar] [CrossRef]
- Poursamar, S.A.; Lehner, A.N.; Azami, M.; Ebrahimi-Barough, S.; Samadikuchaksaraei, A.; Antunes, A.P.M. The effects of crosslinkers on physical, mechanical, and cytotoxic properties of gelatin sponge prepared via in-situ gas foaming method as atissue engineering scaffold. Mater. Sci. Eng. C 2016, 63, 1–9. [Google Scholar] [CrossRef]
- Farris, S.; Song, J.; Huang, Q. Alternative reaction mechanism for the cross-linking of gelatin with glutaraldehyde. J. Agric. Food Chem. 2010, 58, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- An, J.; Teoh, J.E.M.; Suntornnond, R.; Chua, C.K. Design and 3D printing of scaffolds and tissues. Engineering 2015, 1, 261–268. [Google Scholar] [CrossRef]
- Chen, L.; Huang, C.; Zhong, Y.; Chen, Y.; Zhang, H.; Zheng, Z.; Jiang, Z.; Wei, X.; Peng, Y.; Huang, L.; et al. Multifunctional sponge scaffold loaded with concentrated growth factors for promoting wound healing. Iscience 2022, 26, 105835. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lee, J.S.; Kim, J.; Lee, Y.B.; Shin, H.; Um, S.H.; Kim, J.B.; Park, K.I.; Lee, H.; Cho, S.W. Polydopamine-mediated surface modification of scaffold materials for human neural stem cellengineering. Biomaterials 2012, 33, 6952–6964. [Google Scholar] [CrossRef]
- Lv, H.; Li, L.; Sun, M.; Zhang, Y.; Chen, L.; Rong, Y.; Li, Y. Mechanism of regulation of stem cell differentiation by matrix stiffness. Stem Cell Res. Ther. 2015, 6, 103. [Google Scholar] [CrossRef]
- Fraley, S.I.; Feng, Y.; Krishnamurthy, R.; Kim, D.-H.; Celedon, A.; Longmore, G.D.; Wirtz, D. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol. 2010, 12, 598. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yerra, P.; Migliario, M.; Gino, S.; Sabbatini, M.; Bignotto, M.; Invernizzi, M.; Renò, F. Polydopamine Blending Increases Human Cell Proliferation in Gelatin–Xanthan Gum 3D-Printed Hydrogel. Gels 2024, 10, 145. https://doi.org/10.3390/gels10020145
Yerra P, Migliario M, Gino S, Sabbatini M, Bignotto M, Invernizzi M, Renò F. Polydopamine Blending Increases Human Cell Proliferation in Gelatin–Xanthan Gum 3D-Printed Hydrogel. Gels. 2024; 10(2):145. https://doi.org/10.3390/gels10020145
Chicago/Turabian StyleYerra, Preetham, Mario Migliario, Sarah Gino, Maurizio Sabbatini, Monica Bignotto, Marco Invernizzi, and Filippo Renò. 2024. "Polydopamine Blending Increases Human Cell Proliferation in Gelatin–Xanthan Gum 3D-Printed Hydrogel" Gels 10, no. 2: 145. https://doi.org/10.3390/gels10020145
APA StyleYerra, P., Migliario, M., Gino, S., Sabbatini, M., Bignotto, M., Invernizzi, M., & Renò, F. (2024). Polydopamine Blending Increases Human Cell Proliferation in Gelatin–Xanthan Gum 3D-Printed Hydrogel. Gels, 10(2), 145. https://doi.org/10.3390/gels10020145