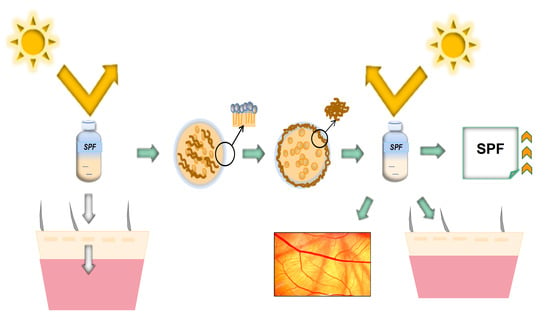
Anti-UV Microgel Based on Interfacial Polymerization to Decrease Skin Irritation of High Permeability UV Absorber Ethylhexyl Methoxycinnamate
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Characterization of EHMC-pEDGMA Microgel
2.1.1. The SEM of EHMC-pEDGMA Microgel
2.1.2. The PSD Measurement of EHMC-pEDGMA Microgel
2.1.3. FTIR Spectroscope
2.1.4. Contact Angle Test
2.1.5. The Thermogravimetric Analysis of the Sunscreen Microgel
2.2. The Performance of EHMC-pEDGMA Microgel
2.2.1. Skin Penetration
2.2.2. Skin Irritation Assays
2.2.3. Sun Protection Factor
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of EHMC-pEDGMA Microgel
4.2.2. Morphological Observation and Particle Size
4.2.3. Fourier Infrared Spectroscopy (FTIR) Analysis
4.2.4. Contact Angle Analysis
4.2.5. Thermogravimetric Analysis
4.2.6. Skin Permeation Behavior
4.2.7. Skin Irritation Assays
4.2.8. Sun Protection Factor
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, S.; Ho, J.C.S.; Teo, D.L.C.; Gupta, S.; Nallani, M. Biomimetic Stratum Corneum Liposome Models: Lamellar Organization and Permeability Studies. Membranes 2023, 13, 135. [Google Scholar] [CrossRef]
- D’Angelo Costa, G.M.; Maia Campos, P.M.B.G. Development of Photoprotective Formulations: Influence of Formulation Composition on the SPF and Mechanical Properties. AAPS PharmSciTech 2023, 24, 97. [Google Scholar] [CrossRef]
- Hiller, J.; Klotz, K.; Meyer, S. Systemic availability of lipophilic organic UV filters through dermal sunscreen exposure. Environ. Int. 2019, 132, 105068. [Google Scholar] [CrossRef]
- Manová, E.; von Goetz, N.; Hungerbuehler, K. Aggregate consumer exposure to UV filter ethylhexyl methoxycinnamate via personal care products. Environ. Int. 2015, 74, 249–257. [Google Scholar] [CrossRef]
- Oral, D.; Yirun, A.; Erkekoglu, P. Safety Concerns of Organic Ultraviolet Filters: Special Focus on Endocrine-Disrupting Properties. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 201–212. [Google Scholar] [CrossRef]
- Lorigo, M.; Cairrao, E. UV-B filter octylmethoxycinnamate-induced vascular endothelial disruption on rat aorta: In silico and in vitro approach. Chemosphere 2022, 307 Pt 2, 135807. [Google Scholar] [CrossRef]
- Huang, Y.R.; Japhet, L.; Zhao, Y.Y. Fate of UV filter Ethylhexyl methoxycinnamate in rat model and human urine: Metabolism, exposure and demographic associations. Sci. Total Environ. 2019, 686, 729–736. [Google Scholar] [CrossRef]
- Guy, B.; Séphanie, A.; Nicolas, O.; Gernot, K.; Anne-Pascale, L. Different damage response of cis and trans isomers of commonly used UV filter after the exposure on adult human liver stem cells and human lymphoblastoid cells: A response. Sci. Total Environ. 2018, 625, 262–263. [Google Scholar] [CrossRef]
- Ao, J.J.; Yuan, T.; Gao, L.; Yu, X.D.; Zhao, X.D.; Tian, Y.; Ding, W.J.; Ma, Y.N. Organic UV filters exposure induces the production of inflammatory cytokines in human macrophages. Sci. Total Environ. 2018, 635, 926–935. [Google Scholar] [CrossRef]
- Perugini, P.; Simeoni, S.; Scalia, S.; Genta, I. Effect of nanoparticle encapsulation on the photostability of the sunscreen agent, 2-ethylhexyl-p-methoxycinnamate. Int. J. Pharm. 2002, 246, 37–45. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M. Encapsulation of the UV filters ethylhexyl methoxycinnamate and butyl methoxydibenzoylmethane in lipid microparticles: Effect on in vivo human skin permeation. Ski. Pharmacol. Physiol. 2011, 24, 182–189. [Google Scholar] [CrossRef]
- Pey-Shiuan, W.; Huang, L.N.; Guo, Y.C. Effects of the novel poly(methyl methacrylate) (PMMA)-encapsulated organic ultraviolet (UV) filters on the UV absorbance and in vitro sun protection factor (SPF). J. Photochem. Photobiol. B Biol. 2014, 131, 24–30. [Google Scholar] [CrossRef]
- Wu, P.S.; Lee, Y.C.; Kuo, Y.C. Development of Octyl Methoxy Cinnamates (OMC)/Silicon Dioxide (SiO2) Nanoparticles by Sol-Gel Emulsion Method. Nanomaterials 2017, 7, 434. [Google Scholar] [CrossRef]
- Song, J.; Chen, S.; Zhao, X.; Cheng, J.B.; Ma, Y.L.; Ren, S.X. Simple, green, ultrasound-assisted preparation of novel core-shell microcapsules from octyl methoxycinnamate and oligomeric proanthocyanidins for UV-stable sunscreen. RSC Adv. 2021, 11, 6374–6382. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Hu, D.; Ragauskas, A.J.; Cao, D.; Liu, W.; Si, C.; Xu, T.; Zhao, P.; Song, X.; et al. Role Evaluation of Active Groups in Lignin on UV-Shielding Performance. ACS Sustain. Chem. Eng. 2022, 10, 11856–11866. [Google Scholar] [CrossRef]
- Suhail, M.; Hsieh, Y.H.; Khan, A.; Minhas, M.U.; Wu, P.C. Preparation and In Vitro Evaluation of Aspartic/Alginic Acid Based Semi-Interpenetrating Network Hydrogels for Controlled Release of Ibuprofen. Gels 2021, 7, 68. [Google Scholar] [CrossRef]
- Inge, A.; William, A.; Andreas, B.; Brändli-Baiocco, A.; Joy, C.; Maggie, D.; Depelchin, B.O.; Armando, R.; Irizarry, R.; Laura, D.M.; et al. PEGylated Biopharmaceuticals. Toxicol. Pathol. 2015, 43, 959–983. [Google Scholar] [CrossRef]
- Faeze, K.A.; Maryam, M.; Unes, S. Layer-by-layer synthesis of the pH-responsive hollow microcapsule and investigation of its drug delivery and anticancer properties. J. Pharm. Sci. 2022, 112, 1072–1080. [Google Scholar] [CrossRef]
- Hu, H.R.; Wang, L.; Xu, B.; Wang, P.; Yuan, J.G.; Yu, Y.Y. Construction of a composite hydrogel of silk sericin via horseradish peroxidase-catalyzed graft polymerization of poly-PEGDMA. J. Biomed. Mater. Research. Part B Appl. Biomater. 2022, 108, 2643–2655. [Google Scholar] [CrossRef]
- Mi, D.; Li, J.J.; Wang, R.J.; Li, Y.K.; Zou, L.; Sun, C.; Yan, S.N.; Yang, H.; Zhao, M.G. Postsurgical wound management and prevention of triple-negative breast cancer recurrence with a pryoptosis-inducing, photopolymerizable hydrogel. J. Control. Release Off. J. Control. Release Soc. 2023, 356, 205–218. [Google Scholar] [CrossRef]
- Singh, G.; Chawla, P.A.; Faruk, A.; Chawla, V.; Kaur, A. Computational Design of Molecularly Imprinted Polymers in Drug Delivery Systems: A Comprehensive Review. Curr. Drug Deliv. 2022, 20, 75–88. [Google Scholar] [CrossRef]
- Kim, S.W.; Pan, C.C. Development of a Dual Hydrogel Model System for Vascularization. Macromol. Biosci. 2020, 20, e2000204. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Ding, J. Controlled Synthesis of Polymers. Chin. J. Chem. 2023, 41, 1235–1248. [Google Scholar] [CrossRef]
- Qiu, L.; Han, X.; Xing, C. Polymerization-Induced Self-Assembly: An Emerging Tool for Generating Polymer-Based Biohybrid Nanostructures. Small Ger. 2023, 19, e2207457. [Google Scholar] [CrossRef]
- Lopes, J.; Fonseca, R.; Viana, T.; Fernandes, C.; Morouço, P.; Moura, C.; Biscaia, S. Characterization of Biocompatible Poly(ethylene glycol)-dimethacrylate Hydrogels for Tissue Engineering. Appl. Mech. Mater. 2019, 890, 290–300. [Google Scholar] [CrossRef]
- Enas, M.A. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Wang, Y. Hairy nanoparticles by atom transfer radical polymerization in miniemulsion. React. Funct. Polym. 2022, 170, 105104. [Google Scholar] [CrossRef]
- Caroline, D.S.M. Exploring the efficacy of ethylene glycol dimethacrylate crosslinked cationised pullulan for gene delivery in cancer cells. J. Drug Deliv. Sci. Technol. 2022, 68, 103067. [Google Scholar] [CrossRef]
- Summonte, S.; Racaniello, G.F.; Lopedota, A.; Denora, N. Thiolated polymeric hydrogels for biomedical application: Cross-linking mechanisms. J. Control. Release 2021, 330, 470–482. [Google Scholar] [CrossRef]
- Elhachem, M.; BouMaroun, E.; Abboud, M.; Maroun Richard, G. Combination of Screen-Printed Carbon Electrode and Molecularly Imprinted Polymers for the Selective Determination of Phenolic Compounds in Wine. Antioxidants 2022, 11, 2036. [Google Scholar] [CrossRef]
- Choi, Y.R.; Chang, Y.H. Microencapsulation of gallic acid through the complex of whey protein concentrate-pectic polysac-charide extracted from Ulmus davidiana. Food Hydrocoll. 2018, 85, 222–228. [Google Scholar] [CrossRef]
- Anwer, M.K.; Al-Mansoor, M.A.; Jamil, S.; Al-Shdefat, R.; Ansari, M.N.; Shakeel, F. Development and evaluation of PLGA polymer based nanoparticles of quercetin. Int. J. Biol. Macromol. 2016, 92, 213–219. [Google Scholar] [CrossRef]
- Yoosefi, S.; Esfandyari-Manesh, M.; Ghorbani-Bidkorpeh, F.; Ahmadi, M.; Moraffah, F.; Dinarvand, R. Novel biodegradable molecularly imprinted polymer nanoparticles for drug delivery of methotrexate anti-cancer; synthesis, characterization and cellular studies. DARU J. Pharm. Sci. 2022, 30, 289–302. [Google Scholar] [CrossRef]
- Savin, C.L.; Popa, M.; Delaite, C.; Costuleanu, M.; Costin, D.; Peptu, C.A. Chitosan grafted-poly(ethylene glycol) methacrylate nanoparticles as carrier for controlled release of bevacizumab. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 843–860. [Google Scholar] [CrossRef]
- Goh, E.G.; Xu, X.; McCormick, P.G. Effect of particle size on the UV absorbance of zinc oxide nanoparticles. Scr. Mater. 2014, 78–79, 49–52. [Google Scholar] [CrossRef]
- Wongngam, Y.; Supanakorn, G.; Thiramanas, R.; Polpanich, D. Smaller Is Not Always Better: Large-Size Hollow Polydopamine Particles Act as an Efficient Sun Protection Factor Booster for Sunscreens. ACS Biomater. Sci. Eng. 2021, 7, 3114–3122. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Campo, A.D.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO–TiO2 composites. Bol. Soc. Esp. Ceram. Y Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Yu, H.; Xue, C.; Qin, Y.; Wen, Y.; Zhang, L.; Li, Y. Preparation and performance of green targeted microcapsules encapsulating surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126733. [Google Scholar] [CrossRef]
- Figueroa-Enriquez, C.E.; Rodríguez-Félix, F.; Plascencia-Jatomea, M.; Sánchez-Escalante, A.; Vargas-López, J.M.; Tapia-Hernández, J.A.; Canizales-Rodríguez, D.F.; Castro-Enriquez, D.D.; Ruiz-Cruz, S.; Santos-Sauceda, I.; et al. Nanoparticles of Betalain-Gelatin with Antioxidant Properties by Coaxial Electrospraying: Preparation and Characterization. ACS Omega 2023, 8, 41156–41168. [Google Scholar] [CrossRef]
- Nakamura, E.; Iwase, H.; Arima-Osonoi, H.; Sakuragi, M. Effect of water content on stratum corneum penetration mechanism of W/O type microemulsions. RSC Adv. 2023, 13, 17742–17749. [Google Scholar] [CrossRef]
- Sharma, A.; Bányiová, K.; Vrana, B.; Justan, I.; Čupr, P. Investigation of cis-trans isomer dependent dermatotoxicokinetics of UV filter ethylhexyl methoxycinnamate through stratum corneum in vivo. Environ. Sci. Pollut. Res. Int. 2017, 24, 25061–25070. [Google Scholar] [CrossRef]
- Portilho, L.; Aiello, L.M.; Vasques, L.I.; Bagatin, E.; Leonardi, G.R. Effectiveness of sunscreens and factors influencing sun protection: A review. Braz. J. Pharm. Sci. 2022, 58, e20693. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Maia Campos, P.M.B.G.; Calixto, L.S.; Darvin, M.E.; Kröger, M.; Schanzer, S.; Lohan, S.B.; Lademann, J.; Meinke, M.C. Influence of physical-mechanical properties on SPF in sunscreen formulations on ex vivo and in vivo skin. Int. J. Pharm. 2021, 598, 120262. [Google Scholar] [CrossRef]
- Damiani, E.; Puglia, C. Nanocarriers and Microcarriers for Enhancing the UV Protection of Sunscreens: An Overview. J. Pharm. Sci. 2019, 108, 3769–3780. [Google Scholar] [CrossRef]
- Lapidot, N.; Gans, O.; Biagini, F.; Sosonkin, L.; Rottman, C. Advanced Sunscreens: UV Absorbers Encapsulated in Sol-Gel Glass Microcapsules. J. Sol-Gel Sci. Technol. 2003, 26, 67–72. [Google Scholar] [CrossRef]
- Andréo-Filho, N.; Bim, A.V.K.; Kaneko, T.M.; Kitice, N.A.; Haridass, I.N.; Abd, E.; Santos Lopes, P.; Thakur, S.S.; Parekh, H.S.; Roberts, M.S.; et al. Development and Evaluation of Lipid Nanoparticles Containing Natural Botanical Oil for Sun Protection: Characterization and in vitro and in vivo Human Skin Permeation and Toxicity. Ski. Pharmacol. Physiol. 2018, 31, 1–9. [Google Scholar] [CrossRef]
- SN/T 2329-2009; Cosmetic Ocular Irritant and Corrosive HET-CAM Test. Standards Press of China: Beijing, China, 2009.








| Sample | Concentration | IS | ES | Irritant |
|---|---|---|---|---|
| EHMC-pEDGMA microgel | 1% | - | 0 | no irritant |
| 5% | - | 0 | no irritant | |
| 10% | - | 0 | no irritant | |
| 50% | - | 0 | no irritant | |
| 100% | - | 0 | no irritant | |
| EHMC (diluted with C12-15 alkylbenzoate) | 1% | 5.42 | - | moderate irritant |
| 0.1 mol/L NaOH solution | positive control | 17.21 | - | corrosive |
| 0.9% NaCl solution | negative control | 0 | - | no irritant |
| C12-15 alkyl benzoate | solvent control | 0 | - | no irritant |
| Deionized water | solvent control | 0 | - | no irritant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; He, Q.-T.; Chen, Y.-F.; Wang, B.-H.; Xu, W.-Y.; Liu, Q.-L.; Liu, H.-M. Anti-UV Microgel Based on Interfacial Polymerization to Decrease Skin Irritation of High Permeability UV Absorber Ethylhexyl Methoxycinnamate. Gels 2024, 10, 177. https://doi.org/10.3390/gels10030177
Wang W, He Q-T, Chen Y-F, Wang B-H, Xu W-Y, Liu Q-L, Liu H-M. Anti-UV Microgel Based on Interfacial Polymerization to Decrease Skin Irritation of High Permeability UV Absorber Ethylhexyl Methoxycinnamate. Gels. 2024; 10(3):177. https://doi.org/10.3390/gels10030177
Chicago/Turabian StyleWang, Wei, Qi-Tong He, Yin-Feng Chen, Bai-Hui Wang, Wen-Ying Xu, Qing-Lei Liu, and Hui-Min Liu. 2024. "Anti-UV Microgel Based on Interfacial Polymerization to Decrease Skin Irritation of High Permeability UV Absorber Ethylhexyl Methoxycinnamate" Gels 10, no. 3: 177. https://doi.org/10.3390/gels10030177
APA StyleWang, W., He, Q. -T., Chen, Y. -F., Wang, B. -H., Xu, W. -Y., Liu, Q. -L., & Liu, H. -M. (2024). Anti-UV Microgel Based on Interfacial Polymerization to Decrease Skin Irritation of High Permeability UV Absorber Ethylhexyl Methoxycinnamate. Gels, 10(3), 177. https://doi.org/10.3390/gels10030177