Hydrogels Based on Chitosan and Nanoparticles and Their Suitability for Dyes Adsorption from Aqueous Media: Assessment of the Last-Decade Progresses
Abstract
:1. Introduction
2. Results and Discussion
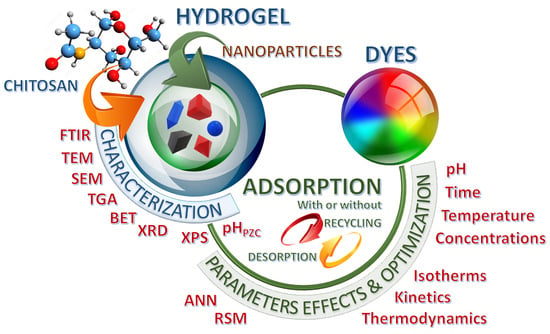
2.1. Examination of the Encompassed Studies
2.2. Synthesis and Charaterization of Hydrogels Based on Chitosan and Nanoparticles
2.3. Dyes Adsorption on Hydrogels Composed of Chitosan and Nanoparticles
| Adsorbent | Dye | Working Conditions * | Removal Efficiency | Adsorption Capacity | Isotherms Models | Kinetics Models | Reference |
|---|---|---|---|---|---|---|---|
| Chitosan/2-mercaptobenzimidazole | Methylene blue | 1. 100 mg/L 2. 0.5–5 g/L 3. 1–11 4. 25–55 °C 5. 0–90 min 6. 50–300 rpm | 92% | 1.28 mmol/g | Langmuir Freundlich Sips | Pseudo-first-order Pseudo-second-order Intraparticle diffusion | [73] |
| Chitosan/clinoptilolite | Methyl orange | 1. 35.34–282.35 mg/L 2. 1–12 g/L 3. 2.2–10 4. 30 °C 5. 0–40 min 6. No stirring | 77.23% | 16.88 mg/g | Langmuir Freundlich Redlich–Peterson Toth Sips | Not available | [74] |
| Chitosan/N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride/cysteine | Methylene blue (MB) Methyl orange (MO) | 1. 0–100 mg/L 2. 2600 mg/L 3. 7 4. 23 °C 5. 0–1440 min 6. No stirring | MB 30% MO 91% | MB 115 mg/g MO 305 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [75] |
| Chitosan/cellulose | Malachite green | 1. 50–200 mg/L 2. 6.66 g/L 3. Natural 4. Ambient 5. 0–45 min 6. No stirring | 98.65% | 115.1 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [76] |
| Chitosan-salicylaldehyde Schiff base/algae/montmorillonite | Remazol brilliant blue R (RBR) Brilliant green (BG) | 1. 20–300 mg/L RBR, 20–200 mg/L BG 2. 0.2–0.8 g/L 3. 4–9 4. Ambient 5. 5–55 min 6. Under stirring | RBR 54.3% BG 79.4% | RBR 148.1 mg/g BG 440.3 mg/g | Freundlich Langmuir | Pseudo-second-order | [77] |
| Iron (III) hydroxide/chitosan | Alizarin red S | 1. 50 mg/L 2. 0.01–0.9 g/L 3. 5 4. 30–80 °C 5. 0–360 min 6. 200 rpm | Not available | 294 mg/g | Langmuir | Pseudo-second-order Pseudo-first-order | [78] |
| EDTA/chitosan/magnetic graphene oxide nano-sheets | Rhodamine B | 1. 50–250 mg/L 2. 0.07–0.18 mg/L 3. 4–9 4. 20–50 °C 5. 5 min 6. Under shaking | 92% | 1085.3 mg/g | Langmuir Freundlich Temkin | Pseudo-second-order Elovich Intraparticle diffusion Pseudo-first-order | [79] |
| Carboxymethyl β-cyclodextrin/nanochitosan/glutaraldehyde | Acid red 37 | 1. 80–214 mg/L 2. 0.16 –1 g/L 3. 2–9.3 4. 20–40 °C 5. 0.33–10 min 6. 250 rpm | 99.6% | 332.60 mg/g | Langmuir Freundlich Temkin Flory–Huggins Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Elovich Intraparticle diffusion | [80] |
| Magnetic polyethyleneimine nanoparticles/sulfonated chitosan/glutaraldehyde. | Methylene blue | 1. 100 μmol/L 2. 2.5 g/L 3. Natural 4. Ambient 5. 0–360 min 6. Under stirring | ≈50% | Not available | Not available | Not available | [81] |
| Gelatin/chitosan/β-cyclodextrin/sodium humate | Methylene blue (MB) Acid fuchsin (AF) | 1. 100–3000 mg/L 2. 1 g/L 3. 2–10 4. 15–50 °C 5. 0–300 min 6. 200 rpm | Not available | MB 1666.7 mg/g AC 714.3 mg/g | Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [82] |
| Graphene oxide/chitosan/magnetic nanoparticles | Sudan I, II, III, IV (SI, SII, SIII, SIV) | 1. 100–400 mg/L 2. 0.1–1 g/L 3. 2–10 4. 15–45 °C 5. 30–120 min 6. 150 rpm | >90% | SI 360.6 mg/g SII 353.7 mg/g SIII 351.0 mg/g SIV 347.6 mg/g | Langmuir Hill Freundlich Temkin Redlich–Peterson | Pseudo-second-order Elovich Pseudo-first-order Intraparticle diffusion | [83] |
| Magnetite nanoparticles/amino-silane/graphene oxide/chitosan/diethylenetriaminepentaacetic acid | Methyl violet | 1. 8–30 mg/L 2. 0.05–3.7 g/L 3. 2–12 4. 13.1–71.9 °C 5. 30–120 min 6. 150 rpm | 94.87% | 243.8 mg/g | Sips Modified Langmuir–Freundlich Langmuir Extended Langmuir Redlich–Peterson Temkin Toth | Pseudo-first-order Pseudo-second-order Elovich Mixed 1, 2-order Pseudo n-order Fractal-like pseudo-first-order Fractal-like pseudo-second-order | [84] |
| Montmorillonite/ chitosan | Calmagite (C) Methylene blue (MB) | 1. 50 mg/L 2. 0.3 g/L 3. 4–10 4. Ambient 5. 0–1800 min 6. No stirring | C ≈ 80% MB 75% | Not available | Not available | Pseudo-second-order | [85] |
| Amphoteric chitosan/gelatin | Acid red 337 | 1. 50–500 mg/L 2. 0.5 g/L 3. 1–10 4. 20–50 °C 5. 1–700 min 6. 100 rpm | 95.6% | ≈750 mg/g | Langmuir Dubinin–Radushkevich Freundlich | Pseudo-second-order Intraparticle diffusion Pseudo-first-order | [86] |
| Gelatin/chitosan/β-cyclodextrin | Malachite green (MG) Crystal violet (CV) Congo red (CR) Methylene blue (MB) Acid fuchsin (AF) Methyl orange (MO) | 1. 100–2800 mg/L 2. 0.5 g/L 3. 2–10 4. 15–50 °C 5. 0–240 min 6. 200 rpm | MG 80% CV 83.3% CR 88.9% MB 85.1% AF 93.3% MO 87.5% | MG not available CV not available CR not available MB 667 mg/g AF 1111 mg/g MO not available | Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Film diffusion | [87] |
| Chitosan/montmorillonite | Methyl orange | 1. 20–320 mg/L 2. 0.32–1.92 mg/cm2 3. 4–11 4. 30–60 °C 5. 1–60 min 6. No stirring | 96.2% | 154.4 mg/g | Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order | [88] |
| Graphene oxide/chitosan | Congo red | 1. 300–600 mg/L 2. 0.25 g/L 3. 3–12 4. 27–60 °C 5. 3–60 min 6. 200 rpm | Not available | 1666 mg/g | Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [89] |
| CaNiFe2O4/ chitosan | Methylene blue | 1. 100–500 mg/L 2. 1–10 g/L 3. 2–12 4. 25–65 °C 5. 5–300 min 6. 400 rpm | Not available | 700 mg/g | Langmuir Freundlich Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [90] |
| Chitosan/β-Cyclodextrin | Indigo carmine | 1. 50–200 mg/L 2. 0.1–1 g/L 3. 3–6 4. 15–50 °C 5. 5–300 min 6. No stirring | ≈100% | 1000 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [91] |
| Tamarind seed-activated carbon/chitosan | Methylene blue (MB) Methyl orange (MO) | 1. 5–50 mg/L 2. 1–3 g/L 3. 2–12 4. Ambient 5. 0–120 min 6. No stirring | Not available | MB 140.62 mg/g MO 94.45 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Elovich | [92] |
| Chitosan/montmorillonite | Reactive red 136 | 1. 50–400 mg/L 2. 0.3–0.6 g/L 3. 3–6 4. 20–50 °C 5. 54–180 min 6. 150 rpm | 74.7% | 445.38 mg/g | Toth Sips Langmuir–Freundlich Langmuir Radke–Prausnitz Dubinin–Radushkevich | Fractal-like mixed 1,2 order Mixed 1,2 order Pseudo-second-order Fractal-like pseudo-2nd-order Fractal-like pseudo-1st-order Pseudo-first-order Elovich | [93] |
| Nano chitosan/activated carbon | Rose Bengal | 1. 1–7 mg/L 2. 1 g/L 3. 6.5–9.5 4. 22–50 °C 5. 0–120 min 6. 150 rpm | 94.7% | Not available | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Liquid film diffusion Intraparticle diffusion | [94] |
| Rice bran/chitosan/aniline (RBCA) Rice bran/chitosan/pyrrole (RBCP) | Malachite green | 1. 5–200 mg/L 2. 0.05–0.3 g/L 3. 2–9 4. 30–60 °C 5. 5–120 min 6. 120 rpm | Not available | RBCA 145.03 mg/g RBCP 55 mg/g | Freundlich Langmuir Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [95] |
| Chitosan/Fe3O4 (MCMs); chitosan/Fe3O4/ [poly(2-(dimethylamino)ethyl methacrylate)] (GMCMs) | Acid green 25 (AG25) Reactive blue 19 (RB19) | 1. 1600 mg/L AG25; 1400 mg/L RB19 2. 1 g/L 3. 4–7 4. 30 °C 5. 0–420 min 6. No stirring | Not available | MCMs AG25 411.9 mg/g RB19 137.8 mg/g GMCMs AG25 961.5 mg/g RB19 691.3 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [96] |
| Chitosan/tripolyphosphate | Basic blue 7 | 1. 50–600 mg/L 2. 0.3–1.2 g/L 3. 2–8 4. 25–55 °C 5. 0.5–390 min 6. No stirring | 100% | 1174 mg/g | Langmuir Fowler–Guggenheim Freundlich Temkin | Pseudo-second-order Pseudo-first-order | [97] |
| Chitosan/Fe3O4/graphene oxide | Eriochrome black T (EBT) Methylene blue (MB) | 1. 250–400 mg/L 2. 1 g/L 3. 2–8 4. 25–45 °C 5. 20–180 min 6. 150 rpm | EBT 86.67% MB 73.33% | EBT 289.85 mg/g MB 261.78 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [98] |
| Chitosan/clinoptilolite | Methyl violet | 1. 25–125 mg/L 2. 0.5–10 g/L 3. 2–9 4. 25–45 °C 5. 5–120 min 6. 250 rpm | Not available | 111.11 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [64] |
| Chitosan/benzil | Reactive orange 16 | 1. 20–200 mg/L 2. 0.02–0.08 g 3. 4–10 4. 25–45 °C 5. 5–25 min 6. 250 rpm | 98.2% | 291.8 mg/g | Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [99] |
| Chitosan/sodium alginate/graphene oxide Chitosan/sodium alginate/graphene oxide/β-cyclodextrin | Rose Bengal | 1. 2–12 mg/L 2. 0.05–0.25 g/L 3. 2–13 4. 30–70 °C 5. 0–660 min 6. Under agitation | >90% | Not available | Langmuir Elovich Freundlich Dubinin–Radushkevich Temkin | Pseudo-second-order Elovich Pseudo-first-order Intraparticle diffusion | [100] |
| Zeolitic imidazole framework/chitosan | Congo red (CR) Malachite green (MG) | 1. 1–50 mg/L 2. 0.02 g/L 3. 4–11 4. 25–45 °C 5. 0–180 min 6. No stirring | Not available | MG 384.6 mg/g CR 500 mg/g | Langmuir Redlich–Peterson Freundlich Temkin | Pseudo-first-order Pseudo-second-order Elovich Intraparticle diffusion | [101] |
| Graphene oxide/chitosan | Reactive black 5 | 1. 100–600 mg/L 2. 0.033–1.66 g/L 3. 2–11 4. 30–70 °C 5. 1–90 min 6. 50–250 rpm | 98.66% | 638.93 mg/g | Langmuir Temkin Freundlich | Pseudo-second-order Pseudo-first-order Elovich Intraparticle diffusion | [102] |
| Rice bran/chitosan | Reactive blue 4 | 1. 200 mg/L 2. 0.5–3.0 g/L 3. 2–10 4. 30–60 °C 5. 0–600 min 6. No stirring | 60% | 57 mg/g | Langmuir Freundlich | Intraparticle diffusion Pseudo-first-order Pseudo-second-order | [103] |
| Chitosan/graphene oxide | Reactive blue 19 | 1. 20–60 mg/L 2. 0.1–1.5 g/L 3. 4–9 4. 20–50 °C 5. 10–120 min 6. No stirring | 99% | Not available | Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [104] |
| Fe3O4/chitosan | Acid blue | 1. 50–1000 mg/L 2. 1 g/L 3. 3–11 4. 30 °C 5. 0–720 min 6. 175 rpm | 80% | 142 mg/g | Langmuir | Pseudo-second-order Pseudo-first-order | [105] |
| Polyethylene glycol-/graphene oxide/chitosan | Methyl orange | 1. 200–25000 mg/L 2. 1 g/L 3. 2–10 4. Ambient 5. 5–90 min 6. 200 rpm | Not available | 150 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [106] |
| Chitosan/cetyltrimethylammonium bromide-aliquat-366 | Tartrazine | 1. 160–3000 mg/L 2. 2 g/L 3. 4–11 4. 25–45 °C 5. 0–45 min 6. No stirring | 90.36% | 45.95 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [107] |
| Chitosan/graphene oxide/copper ferrite | Safranin O (SO) Indigo carmine (IC) | 1. 1 × 10−5–1 × 10−4 M 2. 0.1–0.4 g/L 3. 2–10 4. 20–35 °C 5. 10–100 min 6. 400 rpm | SO 96% IC 95.91% | SO 66.15 mg/g IC 112.6 mg/g | Langmuir Freundlich Temkin Sips Redlich–Peterson | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Elovich | [108] |
| Chitosan/Schiff base pyrano [3,2-c]quinoline-3-carboxaldehyde | Remazol red | 1. 10–100 mg/L 2. 1 g/L 3. 3–11 4. 20–40 °C 5. 0–60 min 6. No stirring | 100% | 344.8 mg/g | Langmuir Temkin Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Elovich | [109] |
| Magnetic calcium/chitosan | Orange II (OII) Methylene blue (MB) | 1. 30–400 mg/L 2. 0.2 g/L 3. 2–11 4. 4–40 °C 5. 0–1440 min 6. No stirring | 90% | OII 492 mg/g MB 350 mg/g | Langmuir Temkin Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [110] |
| N-Guanidinium/chitosan/silica | Methyl orange | 1. 25–2500 mg/L 2. 0.05 g/L 3. 2–9 4. 25 °C 5. 10–160 min 6. No stirring | 95% | 917 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [111] |
| Chitosan/fly ash/Fe3O4 | Reactive orange 16 | 1. 20–150 mg/L 2. 0.04–0.12 g/L 3. 4–10 4. 30–50 °C 5. 0–660 min 6. No stirring | 73.1% | 66.9 mg/g | Freundlich Langmuir Temkin | Pseudo-second-order Pseudo-first-order | [112] |
| Graphene oxide/chitosan/polyvinyl alcohol | Congo red | 1. 10–25 mg/L 2. 1–6 g/L 3. 2–8 4. 25–45 °C 5. 10–150 min 6. 140 rpm | 88.17% | 2.4 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [113] |
| Chitosan/magnetic metal-organic framework | Congo red | 1. 50–600 mg/L 2. 5–30 mg 3. 6–12 4. 25–45 °C 5. 0–250 min 6. 250 rpm | 99.8% | 310.4 mg/g | Liu Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [114] |
| Chitosan/polyvinyl alcohol/MgO/Fe3O4 | Remazol brilliant blue R | 1. 20–240 mg/L 2. 0.2–1 g/L 3. 4–10 4. 30–60 °C 5. 0–180 min 6. No stirring | 63.5% | 163.7 mg/g | Freundlich Langmuir Temkin | Pseudo-second-order Pseudo-first-order | [115] |
| Chitosan/hydroxyapatite | Crystal violet | 1. 10–400 mg/L 2. 0.4–4 g/L 3. 3–12 4. 25–65 °C 5. 5–180 min 6. 100–700 rpm | 93.21% | Not available | Redlich–Peterson Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Elovich | [116] |
| Polypyrrole/chitosan/graphene oxide | Ponceau 4R | 1. 2.4–4.32 mg/L 2. 0.16–0.66 g/L 3. 2–12 4. 30–45 °C 5. 2–210 min 6. 200 rpm | 92.16% | 6.277 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [117] |
| Magnetic halloysite/chitosan | Methylene blue | 1. 20–200 mg/L 2. 1 g/L 3. 5–11 4. 25 °C 5. 0–1440 min 6. 20 rpm | 83.9% | 50.37 mg/g | Langmuir Freundlich Redlich–Peterson | Pseudo-second-order Pseudo-first-order | [118] |
| Chitosan Schiff base (chloroethanoic acid, isopropyl alcohol) | Bismarck brown R (BBR) Eosin Y (EY) | 1. 50–100 mg/L 2. 1–3 g/L 3. 2–10 4. 30–40 °C 5. 10–120 min 6. No stirring | BBR 94.5% EY 99% | BBR 327 mg/g EY 386 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order | [119] |
| Graphene oxide/chitosan/MnO2 | Amido black 10B (AB10) Methylene blue (MB) | 1. 10–200 mg/L 2. 0.6 g/L 3. 2–12 4. 25 °C 5. 0–800 min 6. No stirring | AB10 97% MB 80% | AB10 120 mg/g MB 320 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [120] |
| Iron oxide/polyvinyl alcohol/chitosan combined/activated carbon | Methylene blue | 1. 0.015–0.025 mg/L 2. 0.6 g/L 3. 7 4. Ambient 5. 0–310 h 6. No stirring | Not available | 22.4 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Elovich Intraparticle diffusion | [121] |
| Iron oxide/polyvinyl alcohol/chitosan combined/activated graphite | Methylene blue | 1. 15–25 mg/L 2. 0.6 mg/L 3. 7 4. Ambient 5. 13 days 6. No stirring | 53.962% | 36.385 mg/g | Not available | Pseudo-second-order Pseudo-first-order Elovich | [122] |
| Zinc oxide/chitosan | Eriochrome black T | 1. 20–100 mg/L 2. 0.1–1 g/L 3. 2–12 4. Ambient 5. 0–120 min 6. No stirring | 92% | 40.9 mg/g | Langmuir Freundlich Temkin | Pseudo-first-order Intraparticle diffusion | [123] |
| Kaolin/Chitosan/ TiO2 | Crystal violet | 1. 20–60 mg/L 2. 0.5–2 g/L 3. Natural 4. 25–45 °C 5. 0–180 min 6. 250 rpm | 93.30% | Not available | Freundlich Temkin Langmuir | Pseudo-second-order Pseudo-first-order Elovich Power function | [124] |
| Chitosan/sodium alginate/graphene oxide (CH-ALG-GO); Chitosan/sodium alginate/bentonite (CH-ALG-BN) | Xylenol orange (XO) Methylene blue (MB) | 1. 2–10 mg/L 2. 25 g/L 3. 2–13 4. 30–70 °C 5. 0–540 min 6. No stirring | CH-ALG-GO XO 85% MB 91% CH-ALG-BN XO 93% MB 96% | CH-ALG-GO Not available CH-ALG-BN XO 0.195 mg/g MB 0.731 mg/g | Langmuir Freundlich Elovich Temkin | Pseudo-second-order Elovich Intraparticle diffusion Pseudo-first-order | [125] |
| Chitosan/talc/cloisite 30B clay | Crystal violet (CV) Reactive yellow 145 (RY) | 1. 10–40 mg/L CV; 30–60 mg/L RY 2. 0.1–1.25 g/L 3. 4–10 4. Ambient 5. 0–240 min 6. No stirring | Not available | CV 37.03 mg/g RY 76.9 mg/g | Langmuir Freundlich Dubinin–Radushkevich Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Elovich | [126] |
| Chitosan/hydroxyapatite | Titan yellow (TY) Reactive blue 4 (RB4) | 1. 50–2000 mg/L TY; 50–2500 mg/L RB4 2. 3 g/L 3. 4–10 4. 25–55 °C 5. 0–160 min 6. 300 rpm | Not available | TY 170.7 mg/g RB4 118.4 mg/g | Langmuir Freundlich Sips Extended Langmuir Extended Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [127] |
| Chitosan/poly-(itaconic acid-co-diallyl dimethyl ammonium chloride)/Fe3O4 | Congo red (CR) Methylene blue (MB) | 1. 100–500 mg/L 2. 0.005–0.025 g/L 3. 2–12 4. 25 °C 5. 0–100 min 6. No stirring | Not available | CR 862.06 mg/g MB 1111.11 mg/g | Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [128] |
| Chitosan/silica | Food green 3 | 1. 30–400 mg/L 2. 1–5 g/L 3. 3–9 4. Ambient 5. 5–60 min 6. 600 rpm | 99.31% | 476.19 mg/g | Langmuir Freundlich | Pseudo-second-order | [129] |
| α-ketoglutaric acid Schiff base/chitosan | Congo red | 1. 50–300 mg/L 2. 250–1250 mg/L 3. 3–11 4. 25–55 °C 5. 0–60 min 6. No stirring | 94.87% | 434.78 mg/g | Dubinin–Radushkevich Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Elovich | [130] |
| Kaolin/chitosan | Congo red | 1. 25–200 mg/L 2. 2–12 g/L 3. 4–10 4. 25–55 °C 5. 1–180 min 6. 50–250 rpm | 97% | 104 mg/g | Freundlich Langmuir Dubinin–Radushkevich | Pseudo-first-order Pseudo-second-order Intraparticle diffusion | [131] |
| Chitosan/magnesium oxide | Methyl orange | 1. 10–30 mg/L 2. 0.1–0.5 g/L 3. 6–10 4. 10–50 °C 5. 0–30 min 6. Under agitation | 96.42% | 237.5 mg/g | Langmuir Redlich–Peterson Temkin Freundlich | Pseudo-second-order Pseudo-first-order Elovich Intraparticle diffusion | [132] |
| Chitosan/zero-valent iron | Direct red 81 | 1. 10–50 mg/L 2. 0.05–2 g/L 3. 3–9 4. 15–55 °C 5. 2–12 min 6. No stirring | 97% | Not available | Freundlich Langmuir | Pseudo-first-order Pseudo-second-order | [133] |
| Polyacrylamide-g/chitosan/γ-Fe2O3 | Malachite green | 1. 15–75 g/L 2. 0.5–1.5 g 3. 3–7 4. 25–45 °C 5. 80–210 min 6. No stirring | 73% | 86.28 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich | Pseudo-first-order Pseudo-second-order Elovich Intraparticle diffusion | [134] |
| Chitosan/choline chloride/urea/FeO | Acid blue 80 | 1. 25–250 mg/L 2. 1–7 g/L 3. 3–10 4. 25–45 °C 5. 15–480 min 6. 350 rpm | 99.30% | 61.64 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich Elovich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion Elovich | [135] |
| Chitosan/polypropenoic acid/ethylenediamine/magnetite | Astrazon blue (AB) Lerui acid brilliant blue (LA) | 1. 100–400 mg/L 2. 4 g/L 3. 3–11 4. 25–45 °C 5. 30–1440 min 6. 100 rpm | AB 80% LA 40% | AB 193.21 mg/g LA 51.90 mg/g | Langmuir Freundlich Elovich Temkin Dubinin–Radushkevich Harkin–Jura | Pseudo-first-order Pseudo-second-order Intraparticle diffusion | [136] |
| Diethylenetriamine/chitosan/Fe3O4/Cu | Methyl orange | 1. 10–100 mg/L 2. 0.5 g/L 3. Natural 4. 25 °C 5. 0–40 min 6. No stirring | 96.40% | 144.60 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [137] |
| Chitosan/MgO/Fe3O4 | Reactive blue 19 | 1. 20–350 mg/L 2. 0.2–1 g/L 3. 4–10 4. 30–60 °C 5. 0–180 min 6. 100 rpm | 87.50% | 193.2 mg/g | Freundlich Langmuir Temkin | Pseudo-second-order Pseudo-first-order | [138] |
| Quaternary ammonium magnetic chitosan | Congo red | 1. 50–250 mg/L 2. 0.05–0.35 g/L 3. 2–11 4. 40–60 °C 5. 10–1440 min 6. 130 rpm | 99.8% | 632.80 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [139] |
| Chitosan/dimethyl diallyl ammonium chloride/carboxymethyl cellulose | Malachite green (MG) Rhodamine B (RB) Methylene blue (MB) Bright yellow 7 GL (BY) Methyl orange (MO) Acid blue 113 (AB113) Acid black 172 (AB172) Reactive black 5 (RB5) | 1. 100 mg/L 2. 0.4 g/L 3. 3–11 4. Ambient 5. 0–120 min 6. No stirring | Not available | MG 12.30 mg/g MB 16.90 mg/g RB 21.10 mg/g BY 49.20 mg/g AB172 204.80 mg/g AB113 220.00 mg/g RB5 294.70 mg/g MO 126.50 mg/g | Langmuir Temkin Freundlich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [140] |
| Chitosan/diatomite/calcium alginate | Congo red | 1. 20–150 mg/L 2. 4 g/L 3. 7 4. 20 °C 5. 0–24,000 min 6. No stirring | 89.90% | 38.84 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [141] |
| Chitosan/poly(methacrylate) | Bromocresol green | 1. 5–100 mg/L 2. 2 g/L 3. 1–10 4. Ambient 5. 2–120 min 6. No stirring | 99% | 39.84 mg/g | Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [142] |
| Chitosan/MgO | Reactive blue 19 | 1. 100–700 mg/L 2. 1.33–10.66 g/L 3. 3–9 4. 18–38 °C 5. 30–180 min 6. 150 rpm | 77.62% | 512.82 mg/g | Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [143] |
| Chitosan/silica/Fe3O4 | Methylene blue | 1. 100–550 mg/L 2. 1 g/L 3. 2–9 4. 25 °C 5. 20–160 min 6. Under shaking | 97% | 245 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [144] |
| 2-hydroxy-1-naphthaldehyde linked Fe3O4/chitosan/polyacrylamide | Everzol black | 1. 10–100 mg/L 2. 0.5–2.5 g/L 3. 2–12 4. 10–35 °C 5. 10–20 min 6. No stirring | 94.87% | 63.69 mg/g | Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order | [145] |
| 2-acrylamido-2-methylpropane sulfonic acid/acrylic acid/chitosan/ magnetite | Methylene blue | 1. 100–1200 mg/L 2. 1 g/L 3. 2–11 4. 25–45 °C 5. 0–1440 min 6. 150 rpm | Not available | 925.9 mg/g | Langmuir Freundlich Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [146] |
| Polyvinyl alcohol/ chitosan/silver nanoparticles | Congo red (CR) Crystal violet (CV) | 1. 2–18 mg/L 2. 1 g/L 3. 4–9 4. 25–50 °C 5. 0–2880 min 6. 80 rpm | CR 99.91% CV 94.7% | CR 17.98 mg/g CV 11.365 mg/g | Langmuir Freundlich | Pseudo-first-order Pseudo-second-order | [147] |
| Oxalic acid/chitosan/alumina ceramic | Reactive red 195 | 1. 70–500 mg/L 2. 0.43–4.25 g/L 3. 2–12 4. 25–45 °C 5. 0–1440 min 6. 125 rpm | >80% | 345.3 mg/g | Langmuir Freundlich Temkin Redlich–Peterson Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order Elovich Intraparticle diffusion Mixed surface reaction–diffusion-controlled kinetic | [148] |
| Chitosan/iron oxide | Methylene blue | 1. 10 mg/L 2. 0.4–6 g/L 3. 1–9 4. 25 °C 5. 0–60 min 6. 300 rpm | 80% | 5.12 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich | Pseudo-second-order Elovich Power function Pseudo-first-order | [149] |
| Chitosan/poly (acrylic acid-co-N-isopropylacrylamide)/graphite oxide | Methylene blue (MB) Fuchsin basic (FB) | 1. 100–4000 mg/L 2. 0.13–1.33 g/L 3. Natural 4. 25–45 °C 5. 0–1440 min 6. No stirring | MB 77.7% FB 57.9% | MB 2748.1 mg/g FB 2246.9 mg/g | Redlich–Peterson Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [150] |
| Chitosan/magnetic graphene oxide | Congo red | 1. 150–300 mg/L 2. 2.5–25 g/L 3. 2–11 4. 25–55 °C 5. 0–1440 min 6. 120 rpm | 99.27% | 395.8 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [151] |
| Chitosan/citric acid modified pistachio shell/halloysite nanotubes/glutaraldehyde | Methylene blue | 1. 25–250 mg/L 2. 0.5–3.0 g/L 3. 2–8 4. 25–45 °C 5. 15–240 min 6. 150 rpm | >75% | 111.14 mg/g | Langmuir Freundlich Temkin Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order | [152] |
| Chitosan/laponite | Methylene blue (MB) Congo red (CR) | 1. 100 mg/L 2. 1 g/L 3. 2–10 4. 20–60 °C 5. 0–180 min 6. Under stirring | Not available | MB 563.6 mg/g CR 390.3 mg/g | Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [153] |
| Amino hydroxyapatite/sodium tripolyphosphate/chitosan | Reactive violet 5R | 1. 50–700 mg/L 2. 1.25–10 g/L 3. 3–9 4. 25 °C 5. 5–600 min 6. No stirring | 98% | 365 mg/g | Langmuir Freundlich Temkin | Pseudo-second-order Pseudo-first-order Elovich | [154] |
| Chitosan/tripolyphosphate/zinc oxide | Methyl orange | 1. 150–350 mg/L 2. 1–25 g/L 3. 2–12 4. 25–45 °C 5. 0–210 min 6. No stirring | 99.87% | 185.2 mg/g | Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [155] |
| Sulfonated chitosan/montmorillonite | Methylene blue | 1. 40–400 mg/L 2. 0.2–3 g/L 3. 4–12 4. 30–60 °C 5. 0–180 min 6. 200 rpm | >95% | 141.2 mg/g | Temkin Freundlich Langmuir | Pseudo-second-order Pseudo-first-order | [156] |
| Chitosan/magnetite | Congo red | 1. 250 mg/L 2. 150 mg 3. Natural 4. Ambient 5. 0–120 min 6. Under stirring | Not available | 22.6 mg/g | Not available | Pseudo-second-order Pseudo-first-order | [157] |
| Ethyl acrylate/chitosan | Methylene blue | 1. 20–100 mg/L 2. 0.5–1.5 g/L 3. 7–11 4. 29.2 °C 5. 0–120 min 6. No stirring | 98.4% | 384.61 mg/g | Langmuir Freundlich Temkin | Not available | [158] |
| Chitosan/acrylic acid/acrylamide/bentonite | Malachite green (MG) Methyl violet (MV) | 1. 2.5–20 mg/L MG; 200–1000 mg/L MV 2. Not available 3. 3–10 4. Ambient 5. 0–1440 min 6. Under stirring | MG 93% MV 96.5% | MG 492 mg/g MV 482 mg/g | Langmuir–Freundlich | Pseudo-second-order Pseudo-first-order | [159] |
| Poly(itaconic acid) and poly(acrylic acid) chitosan/magnetite | Methylene blue | 1. 0.1–5 mM 2. 0.167 g/L 3. 7 4. 20 °C 5. 0–1440 min 6. Under shaking | >99% | 470.2 mg/g | Sips Langmuir Freundlich | Pseudo-second-order Pseudo-first-order | [160] |
| Chitosan/glycidyl methacrylate/FeCl3/KPS | Reactive red 120 (RR) Indigo carmine (IC) | 1. 5–600 mg/L 2. 0.0125–0.25 g 3. 3–9 4. 5–50 °C 5. 5–300 min 6. No stirring | RR 100% IV 100% | RR 241 mg/g IC 185 mg/g | Langmuir Freundlich Dubinin–Radushkevich | Pseudo-second-order Pseudo-first-order | [161] |
| Chitosan/Fe3O4 | Reactive black 5 (RB5) Methyl orange (MO) | 1. 25–300 mg/L 2. 3.33 g/L 3. 4–10 4. 25 °C 5. 1–180 min 6. 18 rpm | Not available | RB5 53.02 mg/g MO 70.85 mg/g | Langmuir Freundlich Redlich–Peterson Temkin | Pseudo-second-order Pseudo-first-order Intraparticle diffusion | [162] |
| Magnetite/amino-silica/chitosan/diethylenetriaminepentaacetic acid/graphene oxide | Basic blue 41 | 1. 8–600 mg/L 2. 0.12–2.48 g/L 3. 4–10 4. 17.8–68.2 °C 5. 0–60 min 6. 150 rpm | 95% | 55.87 mg/g | Langmuir–Freundlich Toth Extended Langmuir Temkin Redlich–Peterson Freundlich Langmuir Modified Langmuir | Pseudo-second-order Pseudo-first-order Elovich Mixed 1,2-order Fractal-like pseudo-first-order Fractal-like pseudo-second-order | [163] |
2.4. Adsorption Isotherms
2.5. Kinetic Studies
2.6. Thermodynamic Studies
2.7. Hydrogels’ Reusability
3. Conclusions and Perspectives
- In order to increase the adsorption capacity, it is recommended to modify the hydrogels’ structure by adding new components, such as combinations of clay and metal oxides;
- The efficacy of chitosan nanoparticles-based hydrogels in treating real industrial wastewaters that contain a variety of contaminants besides dyes should be the subject of thorough research;
- It is advised to test the ability of hydrogels to remove an entire panoply of water contaminants at the same time;
- Optimization steps of the hydrogels’ preparation and of the adsorption process by using various methods, such as response surface methodology or artificial neural network, should be compulsorily conducted;
- Further complex investigations should be carried out on the hydrogels’ regeneration and reusability.
4. Methodology
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Altıntıg, E.; Ates, A.; Angın, D.; Topal, Z.; Aydemir, Z. Kinetic, equilibrium, adsorption mechanisms of RBBR and MG dyes on chitosan-coated montmorillonite with an ecofriendly approach. Chem. Eng. Res. Des. 2022, 188, 287–300. [Google Scholar] [CrossRef]
- Chatterjee, S.; Ohemeng-Boahen, G.; Sewu, D.D.; Osei, B.A.; Woo, S.H. Improved adsorption of Congo red from aqueous solution using alkali-treated goethite impregnated chitosan hydrogel capsule. J. Environ. Chem. Eng. 2022, 10, 108244. [Google Scholar] [CrossRef]
- Ahsan, A.; Jamil, F.; Rashad, M.A.; Hussain, M.; Inayat, A.; Akhter, P.; Al-Muhtaseb, A.a.H.; Lin, K.-Y.A.; Park, Y. Wastewater from the textile industry: Review of the technologies for wastewater treatment and reuse. Korean J. Chem. Eng. 2023, 40, 2060–2081. [Google Scholar] [CrossRef]
- Iyyappan, J.; Gaddala, B.; Gnanasekaran, R.; Gopinath, M.; Yuvaraj, D.; Kumar, V. Critical review on wastewater treatment using photo catalytic advanced oxidation process: Role of photocatalytic materials, reactor design and kinetics. Case Stud. Chem. Environ. Eng. 2024, 9, 100599. [Google Scholar] [CrossRef]
- Mukherjee, J.; Lodh, B.K.; Sharma, R.; Mahata, N.; Shah, M.P.; Mandal, S.; Ghanta, S.; Bhunia, B. Advanced oxidation process for the treatment of industrial wastewater: A review on strategies, mechanisms, bottlenecks and prospects. Chemosphere 2023, 345, 140473. [Google Scholar] [CrossRef]
- Saravanan, A.; Deivayanai, V.C.; Kumar, P.S.; Rangasamy, G.; Hemavathy, R.V.; Harshana, T.; Gayathri, N.; Alagumalai, K. A detailed review on advanced oxidation process in treatment of wastewater: Mechanism, challenges and future outlook. Chemosphere 2022, 308, 136524. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.; Soni, B.; Shah, M. A comprehensive review on electro-oxidation and its types for wastewater treatment. Groundw. Sustain. Dev. 2023, 23, 100980. [Google Scholar] [CrossRef]
- Mishra, S.; Sundaram, B. A review of the photocatalysis process used for wastewater treatment. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Rodríguez, S.; Lorenzo, D.; Santos, A.; Romero, A. Comparison of real wastewater oxidation with Fenton/Fenton-like and persulfate activated by NaOH and Fe(II). J. Environ. Manag. 2020, 255, 109926. [Google Scholar] [CrossRef]
- Phan, H.N.Q.; Leu, H.-J.; Nguyen, V.N.D. Enhancing pharmaceutical wastewater treatment: Ozone-assisted electrooxidation and precision optimization via response surface methodology. J. Water Process Eng. 2024, 58, 104782. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; López-Maldonado, E.A.; Pavithra, N.; Méndez-Herrera, P.F.; López-López, J.R.; Baig, U.; Ramamurthy, P.C.; Mubarak, N.M.; Karri, R.R.; et al. Emerging membrane technology and hybrid treatment systems for the removal of micropollutants from wastewater. Desalination 2023, 565, 116873. [Google Scholar] [CrossRef]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-based water and wastewater treatment technologies: Issues, current trends, challenges, and role in achieving sustainable development goals, and circular economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Song, C.; Li, L.; Wang, H.; Pan, Y.; Wang, C.; Li, J.; Wang, T.; Feng, X. Membrane technology coupled with electrochemical advanced oxidation processes for organic wastewater treatment: Recent advances and future prospects. Chem. Eng. J. 2019, 376, 120909. [Google Scholar] [CrossRef]
- Fayyaz Shahandashty, B.; Fallah, N.; Nasernejad, B. Industrial wastewater treatment: Case study on copper removal from colloidal liquid using coagulation. J. Water Process Eng. 2023, 53, 103712. [Google Scholar] [CrossRef]
- Raj, S.; Singh, H.; Bhattacharya, J. Treatment of textile industry wastewater based on coagulation-flocculation aided sedimentation followed by adsorption: Process studies in an industrial ecology concept. Sci. Total Environ. 2023, 857, 159464. [Google Scholar] [CrossRef]
- Abdul Mubarak, N.S.; Chuan, T.W.; Khor, H.P.; Jawad, A.H.; Wilson, L.D.; Sabar, S. Immobilized Fe-loaded chitosan film for Methyl orange dye removal: Competitive ions, reusability, and mechanism. J. Polym. Environ. 2021, 29, 1050–1062. [Google Scholar] [CrossRef]
- Wen, L.; Chen, X.; Chen, C.; Yang, R.; Gong, M.; Zhang, Y.; Fu, Q. Ice-templated porous polymer/UiO-66 monolith for Congo red adsorptive removal. Arab. J. Chem. 2020, 13, 5669–5678. [Google Scholar] [CrossRef]
- Liang, C.-Z.; Sun, S.-P.; Li, F.-Y.; Ong, Y.-K.; Chung, T.-S. Treatment of highly concentrated wastewater containing multiple synthetic dyes by a combined process of coagulation/flocculation and nanofiltration. J. Membr. Sci. 2014, 469, 306–315. [Google Scholar] [CrossRef]
- Xiang, J.; Li, H.; Hei, Y.; Tian, G.; Zhang, L.; Cheng, P.; Zhang, J.; Tang, N. Preparation of highly permeable electropositive nanofiltration membranes using quaternized polyethyleneimine for dye wastewater treatment. J. Water Process Eng. 2022, 48, 102831. [Google Scholar] [CrossRef]
- Kumar, M.S.; Sonawane, S.H.; Bhanvase, B.A.; Bethi, B. Treatment of ternary dye wastewater by hydrodynamic cavitation combined with other advanced oxidation processes (AOP’s). J. Water Process Eng. 2018, 23, 250–256. [Google Scholar] [CrossRef]
- Xia, J.; Marthi, R.; Twinney, J.; Ghahreman, A. A review on adsorption mechanism of gold cyanide complex onto activation carbon. J. Ind. Eng. Chem. 2022, 111, 35–42. [Google Scholar] [CrossRef]
- De Smedt, J.; Heynderickx, P.M.; Arauzo, P.J.; Ronsse, F. Adsorption mechanism of different dyes on chemical activated carbon as quantitative assessment for wastewater treatment: Comparative study between ZnCl2 and its eutectic. Sep. Purif. Technol. 2024, 334, 126002. [Google Scholar] [CrossRef]
- Kshirsagar, D.; Thanekar, P.; Balapure, K.; Bhandari, V.M. Biomass-derived adsorbents and nanocomposites for wastewater treatment. Mater. Today Proc. 2023, 90, 18–29. [Google Scholar] [CrossRef]
- Wang, S.; Lu, W.; Esakkimuthu, S.; Chen, H.; Yang, J.; Mu, M.; Gong, X. Life cycle assessment of carbon-based adsorbent preparation from algal biomass. J. Clean. Prod. 2023, 427, 139269. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, C.; Li, F.; Zhang, X.; Li, M.-C.; Xie, J.; de Hoop, C.F.; Qi, J.; Huang, X. Magnetic nanocellulose-based adsorbent for highly selective removal of Malachite green from mixed dye solution. Int. J. Biol. Macromol. 2023, 253, 126752. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.-Y.; Zhu, W.; Dang, S.; Li, J.-P.; Wu, D.; Li, Y.-h.; Sun, Z.-M. Polyoxometalates-based heterometallic organic–inorganic hybrid materials for rapid adsorption and selective separation of Methylene blue from aqueous solutions. Chem. Commun. 2015, 51, 3336–3339. [Google Scholar] [CrossRef]
- Obsa, A.L.; Shibeshi, N.T.; Mulugeta, E.; Workeneh, G.A. Bentonite/amino-functionalized cellulose composite as effective adsorbent for removal of lead: Kinetic and isotherm studies. Results Eng. 2024, 21, 101756. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Highly efficient Methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles. J. Porous Mater. 2015, 22, 1383–1392. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Surip, S.N.; Alothman, Z.A. Hybrid multifunctional biocomposite of chitosan grafted benzaldehyde/montmorillonite/algae for effective removal of Brilliant green and Reactive blue 19 dyes: Optimization and adsorption mechanism. J. Clean. Prod. 2023, 393, 136334. [Google Scholar] [CrossRef]
- Van Hoa, N.; Minh, N.C.; Cuong, H.N.; Dat, P.A.; Nam, P.V.; Viet, P.H.T.; Phuong, P.T.D.; Trung, T.S. Highly porous hydroxyapatite/graphene oxide/chitosan beads as an efficient adsorbent for dyes and heavy metal ions removal. Molecules 2021, 26, 6127. [Google Scholar] [CrossRef]
- Akbarnejad, S.; Amooey, A.A.; Ghasemi, S. High effective adsorption of Acid fuchsin dye using magnetic biodegradable polymer-based nanocomposite from aqueous solutions. Microchem. J. 2019, 149, 103966. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Liu, Y. Green synthesis of chitosan–phytic acid polymers and nanoparticles. Ind. Crops Prod. 2023, 199, 116747. [Google Scholar] [CrossRef]
- Sayed, A.; Mazrouaa, A.M.; Mohamed, M.G.; Abdel-Raouf, M.E.S. Green synthesis of chitosan/erythritol/graphene oxide composites for simultaneous removal of some toxic species from simulated solution. Environ. Sci. Pollut. Res. 2023, 30, 25903–25919. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene oxide-chitosan composite material for treatment of a model dye effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.L.; Tran, T.T.V.; Juang, R.S.; Nguyen, C.H. Graphene oxide crosslinked chitosan composites for enhanced adsorption of cationic dye from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2023, 142, 104678. [Google Scholar] [CrossRef]
- Wu, R.; Abdulhameed, A.S.; Yong, S.K.; Li, H.; Alothman, Z.A.; Wilson, L.D.; Jawad, A.H. Functionalization of chitosan biopolymer with SiO2 nanoparticles and benzaldehyde via hydrothermal process for Acid red 88 dye adsorption: Box-Behnken design optimization. Int. J. Biol. Macromol. 2023, 247, 125806. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Moneim El-Ghanam, A.; Saad, S.R. Fast and efficient adsorptive capture of Congo red and Erythromycin pollutants by a novel nanobiosorbent from crosslinked nanosilica with nanobiochar and chitosan. Inorg. Chem. Commun. 2023, 158, 111557. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ren, W.; Tang, Q.; Cao, H.; Wang, L.; Zheng, X. Biological preparation of chitosan-loaded silver nanoparticles: Study of Methylene blue adsorption as well as antibacterial properties under light. ACS Omega 2023, 8, 22998–23007. [Google Scholar] [CrossRef]
- Abdelghaffar, F. Biosorption of anionic dye using nanocomposite derived from chitosan and silver nanoparticles synthesized via cellulosic banana peel bio-waste. Environ. Technol. Innov. 2021, 24, 101852. [Google Scholar] [CrossRef]
- Mohammad, A.T.; Abdulhameed, A.S.; Jawad, A.H. Box-Behnken design to optimize the synthesis of new crosslinked chitosan-glyoxal/TiO2 nanocomposite: Methyl orange adsorption and mechanism studies. Int. J. Biol. Macromol. 2019, 129, 98–109. [Google Scholar] [CrossRef]
- Wei, J.; Yan, L.; Zhang, Z.; Hu, B.; Gui, W.; Cui, Y. Carbon nanotube/chitosan hydrogel for adsorption of Acid red 73 in aqueous and soil environments. BMC Chem. 2023, 17, 104. [Google Scholar] [CrossRef]
- Sirajudheen, P.; Karthikeyan, P.; Vigneshwaran, S.; Nikitha, M.; Hassan, C.A.A.; Meenakshi, S. Ce(III) networked chitosan/β-cyclodextrin beads for the selective removal of toxic dye molecules: Adsorption performance and mechanism. Carbohydr. Polym. Technol. Appl. 2020, 1, 100018. [Google Scholar] [CrossRef]
- Solano, M.A.; Galan, J.; Vallejo, W.; Arana, V.A.; Grande-Tovar, C.D. Chitosan beads incorporated with graphene oxide/titanium dioxide nanoparticles for removing an anionic dye. Appl. Sci. 2021, 11, 9439. [Google Scholar] [CrossRef]
- Mahmoud, G.A.; Sayed, A.; Thabit, M.; Safwat, G. Chitosan biopolymer based nanocomposite hydrogels for removal of Methylene blue dye. SN Appl. Sci. 2020, 2, 968. [Google Scholar] [CrossRef]
- Doondani, P.; Jugade, R.; Gomase, V.; Shekhawat, A.; Bambal, A.; Pandey, S. Chitosan/graphite/polyvinyl alcohol magnetic hydrogel microspheres for decontamination of Reactive orange 16 dye. Water 2022, 14, 3411. [Google Scholar] [CrossRef]
- El-Kousy, S.M.; El-Shorbagy, H.G.; El-Ghaffar, M.A.A. Chitosan/montmorillonite composites for fast removal of Methylene blue from aqueous solutions. Mater. Chem. Phys. 2020, 254, 123236. [Google Scholar] [CrossRef]
- Kloster, M.; de Almeida, A.A.; Muraca, D.; Marcovich, N.E.; Mosiewicki, M.A. Chitosan-based magnetic particles as adsorbents for anionic contaminants. Eng. Sci. 2023, 22, 851. [Google Scholar] [CrossRef]
- Nandanwar, P.; Jugade, R.; Gomase, V.; Shekhawat, A.; Bambal, A.; Saravanan, D.; Pandey, S. Chitosan-Biopolymer-Entrapped Activated charcoal for adsorption of Reactive orange dye from aqueous phase and CO2 from gaseous phase. J. Compos. Sci. 2023, 7, 103. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Jawad, A.H.; Ridwan, M.; Khadiran, T.; Wilson, L.D.; Yaseen, Z.M. Chitosan/carbon-doped TiO2 composite for adsorption of two anionic fyes in solution and gaseous SO2 capture: Experimental modeling and optimization. J. Polym. Environ. 2022, 30, 4619–4636. [Google Scholar] [CrossRef]
- Kaur, K.; Jindal, R. Comparative study on the behaviour of chitosan-gelatin based hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr. Polym. 2019, 207, 398–410. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Alhumaimess, M.S.; Alqadami, A.A.; Hassan, H.M.A.; Chen, Q.; Alamri, M.S.; Alanzi, M.M.J.; Alraddadi, T.S. Chitosan-carboxylic acid grafted multifunctional magnetic nanocomposite as a novel adsorbent for effective removal of Methylene blue dye from aqueous environment. Chem. Eng. Sci. 2023, 280, 119017. [Google Scholar] [CrossRef]
- Alshahrani, A.A.; Alorabi, A.Q.; Hassan, M.S.; Amna, T.; Azizi, M. Chitosan-functionalized hydroxyapatite-cerium oxide heterostructure: An efficient adsorbent for dyes removal and antimicrobial agent. Nanomaterials 2022, 12, 2713. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; Arafa, W.A.A.; Ahmed, I.M.; El-Shanshory, A.A.; Abu-Saied, M.A.; Soliman, H.M.A.; Abdelgawad, M.A.; Ali, H.M.; Bräse, S. Chitosan-functionalized-graphene oxide (GO@CS) beads as an effective adsorbent to remove cationic dye from wastewater. Polymers 2022, 14, 4236. [Google Scholar] [CrossRef]
- Lu, F.; Ding, G.; Ma, X.; Huang, B.; You, L. Chitosan-gelatin/cetyltrimethylammonium bromide magnetic polymer composites as reusable high performance adsorbent for AR 18 removal. J. Mol. Liq. 2023, 391, 123303. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, C.; He, J.; Peng, W.; Cao, Y.; Liu, J.; Huang, Y.; Fan, G. Chitosan-induced self-assembly of montmorillonite nanosheets along the end-face for Methylene blue removal from water. Int. J. Biol. Macromol. 2023, 227, 952–961. [Google Scholar] [CrossRef]
- Kurczewska, J. Chitosan-montmorillonite hydrogel beads for effective dye adsorption. J. Water Process Eng. 2022, 48, 102928. [Google Scholar] [CrossRef]
- Iovescu, A.; Stîngă, G.; Maxim, M.E.; Gosecka, M.; Basinska, T.; Slomkowski, S.; Angelescu, D.; Petrescu, S.; Stănică, N.; Băran, A.; et al. Chitosan-polyglycidol complexes to coating iron oxide particles for dye adsorption. Carbohydr. Polym. 2020, 246, 116571. [Google Scholar] [CrossRef]
- Blachnio, M.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A.; Nosach, L.V.; Voronin, E.F. Chitosan–silica composites for adsorption application in the treatment of water and wastewater from anionic dyes. Int. J. Mol. Sci. 2023, 24, 11818. [Google Scholar] [CrossRef]
- Shah, A.; Arjunan, A.; Baroutaji, A.; Zakharova, J. A review of physicochemical and biological contaminants in drinking water and their impacts on human health. Water Sci. Eng. 2023, 16, 333–344. [Google Scholar] [CrossRef]
- Benson, R.; Conerly, O.D.; Sander, W.; Batt, A.L.; Boone, J.S.; Furlong, E.T.; Glassmeyer, S.T.; Kolpin, D.W.; Mash, H.E.; Schenck, K.M.; et al. Human health screening and public health significance of contaminants of emerging concern detected in public water supplies. Sci. Total Environ. 2017, 579, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Santillán, F.; Mejía, I.M.M.; Goicoechea, H.C. Comparative study of uncoated and tetraethylorthosilicate-coated magnetic chitosan beads in the adsorption of two textile dyes. Int. J. Environ. Sci. Technol. 2023, 20, 11821–11836. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Jawad, A.H.; Vigneshwaran, S.; Alothman, Z.A.; Yaseen, Z.M. Different TiO2 phases (Degussa/Anatase) modified cross-linked chitosan composite for the removal of Reactive red 4 dye: Box–Behnken Design. J. Polym. Environ. 2022, 30, 5084–5099. [Google Scholar] [CrossRef]
- Altıntıg, E.; Kabadayı, O.; Bozdag, D.; Altundag, S.; Altundag, H. Artificial neural network mathematical modeling of Methyl violet removal with chitosan-coated clinoptilolite. Desalin. Water Treat. 2022, 250, 252–265. [Google Scholar] [CrossRef]
- Sadiq, A.C.; Olasupo, A.; Rahim, N.Y.; Ngah, W.S.W.; Suah, F.B.M. Comparative removal of Malachite green dye from aqueous solution using deep eutectic solvents modified magnetic chitosan nanoparticles and modified protonated chitosan beads. J. Environ. Chem. Eng. 2021, 9, 106281. [Google Scholar] [CrossRef]
- Simonescu, C.M.; Tătăruş, A.; Culiţă, D.C.; Stănică, N.; Ionescu, I.A.; Butoi, B.; Banici, A.M. Comparative study of CoFe2O4 nanoparticles and CoFe2O4-chitosan composite for Congo red and Methyl orange removal by adsorption. Nanomaterials 2021, 11, 711. [Google Scholar] [CrossRef]
- Muangrak, W.; Thouchprasitchai, N.; Phongboonchoo, Y.; Pongstabodee, S. Dual functional composite of montmorillonite-rich/chitosan (Mcc) for decolorizing the water used in joss paper process: Thermodynamic, isotherm, and kinetic studies. Appl. Sci. 2020, 10, 7493. [Google Scholar] [CrossRef]
- Patel, S.R.; Patel, R.H.; Patel, M.P. Eco-friendly bioadsorbent-based polymer composites as a pH-responsive material for selective removal of anionic and azo dyes from aqueous solutions. J. Macromol. Sci. Part A Pure Appl. Chem. 2020, 58, 97–110. [Google Scholar] [CrossRef]
- Coura, J.C.; Profeti, D.; Profeti, L.P.R. Eco-friendly chitosan/quartzite composite as adsorbent for dye removal. Mater. Chem. Phys. 2020, 256, 123711. [Google Scholar] [CrossRef]
- Da Silva, R.C.; de Aguiar, S.B.; da Cunha, P.L.R.; de Paula, R.C.M.; Feitosa, J.P.A. Effect of microwave on the synthesis of polyacrylamide-g-chitosan gel for azo dye removal. React. Funct. Polym. 2020, 148, 104491. [Google Scholar] [CrossRef]
- Al-Harby, N.F.; Almarshed, M.S.; Mohamed, N.A. Effect of single-walled carbon nanotubes on the adsorption of Basic red 12 dye by trimellitic anhydride isothiocyanate-crosslinked chitosan hydrogel. Cellul. Chem. Technol. 2023, 57, 445–458. [Google Scholar] [CrossRef]
- Vakili, M.; Zwain, H.M.; Mojiri, A.; Wang, W.; Gholami, F.; Gholami, Z.; Giwa, A.S.; Wang, B.; Cagnetta, G.; Salamatinia, B. Effective adsorption of Reactive black 5 onto hybrid hexadecylamine impregnated chitosan-powdered activated carbon beads. Water 2020, 12, 2242. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Elgarahy, A.M.; Al-Bogami, A.S.; Hamza, M.F.; Guibal, E. 2-Mercaptobenzimidazole-functionalized chitosan for enhanced removal of Methylene blue: Batch and column studies. J. Environ. Chem. Eng. 2021, 9, 105609. [Google Scholar] [CrossRef]
- Babazadeh, M.; Irannezhad, M.; Abolghasemi, H.; Hosseiniyan, S.B.; Ehsani, A. 3D mathematical modeling of external mass transfer effect in high-rate adsorption process. Surf. Interfaces 2022, 29, 101771. [Google Scholar] [CrossRef]
- Medeiros Borsagli, F.G.L. A green 3D scaffolds based on chitosan with thiol group as a model for adsorption of hazardous organic dye pollutants. Desalin. Water Treat. 2019, 169, 395–411. [Google Scholar] [CrossRef]
- Shetty, B.; Yashodha, S.R.; Johns, J. A green approach to the removal of Malachite green dye from aqueous medium using chitosan/cellulose blend. Fibers Polym. 2023, 24, 1297–1307. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Surip, S.N.; Alothman, Z.A. A new matrix of chitosan-salicylaldehyde Schiff base/algae/montmorillonite for adsorption of anionic and cationic dyes: Statistical optimization and adsorption mechanism. J. Polym. Environ. 2023, 31, 3768–3782. [Google Scholar] [CrossRef]
- Limchoowong, N.; Sricharoen, P.; Chanthai, S. A novel bead synthesis of the Chiron-sodium dodecyl sulfate hydrogel and its kinetics-thermodynamics study of superb adsorption of Alizarin red S from aqueous solution. J. Polym. Res. 2019, 26, 265. [Google Scholar] [CrossRef]
- Nekouei Marnani, N.; Shahbazi, A. A novel environmental-friendly nanobiocomposite synthesis by EDTA and chitosan functionalized magnetic graphene oxide for high removal of Rhodamine B: Adsorption mechanism and separation property. Chemosphere 2019, 218, 715–725. [Google Scholar] [CrossRef]
- Elbadawy, H.A.; El-Dissouky, A.; Hussein, S.M.; El-Kewaey, S.R.; Elfeky, S.A.; El-Ghannam, G. A novel terpolymer nanocomposite (carboxymethyl β-cyclodextrin–nano chitosan–glutaraldehyde) for the potential removal of a textile dye Acid red 37 from water. Front. Chem. 2023, 11, 1115377. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, X.; Wang, Y.; Sun, S.; Zhao, C. A recyclable and regenerable magnetic chitosan absorbent for dye uptake. Carbohydr. Polym. 2016, 150, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, R.; Wang, X.; Li, M.; Yang, W. A superabsorbent hydrogel for removal of dyes from aqueous solution. J. Polym. Environ. 2022, 30, 3327–3339. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A.; Daniel, A.B. A sustainable nanocomposite, graphene oxide bi-functionalized with chitosan and magnetic nanoparticles for enhanced removal of Sudan dyes. J. Dispers. Sci. Technol. 2023, 44, 806–818. [Google Scholar] [CrossRef]
- Asadabadi, S.; Merati, Z. A tailored magnetic composite synthesized by graphene oxide, chitosan and aminopolycarboxylic acid for diminishing dye contaminant. Cellulose 2021, 28, 2327–2351. [Google Scholar] [CrossRef]
- Geuna, A.; Alvarez, M.; Satti, A.J. Adsorbent composites of montmorillonite and chitosan of different molecular weight, obtained by gamma irradiation. J. Environ. Chem. Eng. 2022, 10, 107080. [Google Scholar] [CrossRef]
- He, B.; Xue, H. Adsorption behaviors of acid dye by amphoteric chitosan/gelatin composite microspheres. Water Qual. Res. J. 2015, 50, 314–325. [Google Scholar] [CrossRef]
- Ren, J.; Li, M.; Wang, X.; Li, R.; Wang, H.; Yang, W. Adsorption behaviors of dyes on a biodegradable gelatin/chitosan/β-cyclodextrin hydrogel from an aqueous solution. Colloid Polym. Sci. 2022, 300, 785–800. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A.; Jawad, A.H.; Sabar, S. Adsorption characteristics and mechanistic study of immobilized chitosan-montmorillonite composite for Methyl orange removal. J. Polym. Environ. 2020, 28, 1901–1913. [Google Scholar] [CrossRef]
- Mizhir, A.A.; Abdulwahid, A.A.; Al-Lami, H.S. Adsorption of carcinogenic dye Congo red onto prepared graphene oxide-based composites. Desalin. Water Treat. 2020, 202, 381–395. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Mubarak, M.F. Adsorption of cationic dye using a newly synthesized CaNiFe2O4/chitosan magnetic nanocomposite: Kinetic and isotherm studies. J. Polym. Environ. 2021, 29, 1835–1851. [Google Scholar] [CrossRef]
- Kekes, T.; Tzia, C. Adsorption of Indigo carmine on functional chitosan and β-cyclodextrin/chitosan beads: Equilibrium, kinetics and mechanism studies. J. Environ. Manag. 2020, 262, 110372. [Google Scholar] [CrossRef] [PubMed]
- Kamdod, A.S.; Pavan Kumar, M.V. Adsorption of Methylene blue and Methyl orange on tamarind seed activated carbon and its composite with chitosan: Equilibrium and kinetic studies. Desalin. Water Treat. 2022, 252, 408–419. [Google Scholar] [CrossRef]
- Li, J.; Cai, J.; Zhong, L.; Cheng, H.; Wang, H.; Ma, Q. Adsorption of Reactive red 136 onto chitosan/montmorillonite intercalated composite from aqueous solution. Appl. Clay Sci. 2019, 167, 9–22. [Google Scholar] [CrossRef]
- Hassan, M.G.; Wassel, M.A.; Gomaa, H.A.; Elfeky, A.S. Adsorption of Rose Bengal dye from waste water onto modified biomass. Sci. Rep. 2023, 13, 14776. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Jabeen, A.; Iqbal, M.; Noreen, S.; Naseem, Z. Adsorptive behavior of rice bran-based composites for Malachite green dye: Isotherm, kinetic and thermodynamic studies. J. Mol. Liq. 2017, 237, 322–333. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, H.; Zhou, H.; Wang, Y.; Luo, K.; Zhao, C.; Peng, Y.; Zheng, X. Adsorptive removal of anionic dyes by chitosan-based magnetic microspheres with pH-responsive properties. J. Mol. Liq. 2018, 256, 424–432. [Google Scholar] [CrossRef]
- Morais da Silva, P.M.; Camparotto, N.G.; Grego Lira, K.T.; Franco Picone, C.S.; Prediger, P. Adsorptive removal of basic dye onto sustainable chitosan beads: Equilibrium, kinetics, stability, continuous-mode adsorption and mechanism. Sustain. Chem. Pharm. 2020, 18, 100318. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Abouelreash, Y.G.; AlReshaidan, S.; Naglah, A.M. Application of novel modified chitosan hydrogel composite for the efficient removal of Eriochrome black T and Methylene blue dyes from aqueous media. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1142–1158. [Google Scholar] [CrossRef]
- Normi, N.I.; Abdulhameed, A.S.; Surip, S.N.; Alothman, Z.A.; Wilson, L.D.; Jawad, A.H. Benzil Schiff base side-chain polymer-crosslinked chitosan via hydrothermal process for Reactive orange 16 dye removal: An optimized and comparative study with chitosan. J. Polym. Environ. 2023, 31, 1986–2004. [Google Scholar] [CrossRef]
- Jindal, R. β-Cyclodextrin mediated efficient removal of Rose Bengal using chitosan/sodium alginate/graphene oxide nanocomposite: A comparative study. Iran. Polym. J. (Engl. Ed.) 2022, 31, 931–948. [Google Scholar] [CrossRef]
- Amin, P.; Shojaei, A.; Hamzehlouyan, T. ZIF-8/Chitosan hybrid nanoparticles with tunable morphologies as superior adsorbents towards both anionic and cationic dyes for a broad range of acidic and basic environments. Microporous Mesoporous Mater. 2022, 343, 112149. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Yang, T.C.K.; Pan, G.T.; Thangalazhy-Gopakumar, S.; Gan, S. Utilisation of eco-friendly and low cost 3D graphene-based composite for treatment of aqueous Reactive black 5 dye: Characterisation, adsorption mechanism and recyclability studies. J. Taiwan Inst. Chem. Eng. 2020, 114, 57–66. [Google Scholar] [CrossRef]
- Hong, G.B.; Yu, T.J.; Lee, H.C.; Ma, C.M. Using rice bran hydrogel beads to remove dye from aqueous solutions. Sustainability 2021, 13, 5640. [Google Scholar] [CrossRef]
- Mohamadi, M.B.; Ejazi, H.; Azadbakht, F. Using composite chitosan-graphene oxide to eliminate Reactive blue 19 from water solutions: The study of adsorption kinetics and reaction thermodynamics. Desalin. Water Treat. 2019, 155, 341–349. [Google Scholar] [CrossRef]
- Xu, P.; Zheng, M.; Chen, N.; Wu, Z.; Xu, N.; Tang, J.; Teng, Z. Uniform magnetic chitosan microspheres with radially oriented channels by electrostatic droplets method for efficient removal of Acid blue. J. Taiwan Inst. Chem. Eng. 2019, 104, 210–218. [Google Scholar] [CrossRef]
- Pervez, M.N.; Jahid, M.A.; Mishu, M.M.R.; Talukder, M.E.; Buonerba, A.; Jiang, T.; Liang, Y.; Tang, S.; Zhao, Y.; Dotto, G.L.; et al. Tuning the surface functionality of polyethylene glycol-modified graphene oxide/chitosan composite for efficient removal of dye. Sci. Rep. 2023, 13, 13460. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, S.; Ayati, A.; Tanhaei, B.; Al-Othman, A.; Karimi, F. The surfactant-ionic liquid bi-functionalization of chitosan beads for their adsorption performance improvement toward Tartrazine. Environ. Res. 2022, 204, 111961. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Ahmed, M.A.; Mohamed, A.A. Synthesis, characterization and application of chitosan/graphene oxide/copper ferrite nanocomposite for the adsorptive removal of anionic and cationic dyes from wastewater. RSC Adv. 2023, 13, 5337–5352. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Hassanin, H.M.; Abdelrazek, M.M. Synthesis, and characterization of chitosan bearing pyranoquinolinone moiety for textile dye adsorption from wastewater. Water Sci. Technol. 2020, 81, 421–435. [Google Scholar] [CrossRef]
- He, C.; Shi, L.; Lou, S.; Liu, B.; Zhang, W.; Zhang, L. Synthesis of spherical magnetic calcium modified chitosan micro-particles with excellent adsorption performance for anionic-cationic dyes. Int. J. Biol. Macromol. 2019, 128, 593–602. [Google Scholar] [CrossRef]
- Salama, A.; Hesemann, P. Synthesis of N-guanidinium-chitosan/silica hybrid composites: Efficient adsorbents for anionic pollutants. J. Polym. Environ. 2018, 26, 1986–1997. [Google Scholar] [CrossRef]
- Jawad, A.H.; Malek, N.N.A.; Abdulhameed, A.S.; Razuan, R. Synthesis of magnetic chitosan-fly ash/Fe3O4 composite for adsorption of Reactive orange 16 dye: Optimization by Box–Behnken Design. J. Polym. Environ. 2020, 28, 1068–1082. [Google Scholar] [CrossRef]
- Das, L.; Das, P.; Bhowal, A.; Bhattachariee, C. Synthesis of hybrid hydrogel nano-polymer composite using graphene oxide, chitosan and PVA and its application in waste water treatment. Environ. Technol. Innov. 2020, 18, 100664. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, X.; Shao, M.; Fang, L.; Zhao, X.; Xu, J.; Wang, X. Synthesis of chitosan-modified magnetic metal-organic framework and its adsorption of Congo red and antibacterial activity. Microporous Mesoporous Mater. 2022, 342, 112042. [Google Scholar] [CrossRef]
- Jawad, A.H.; Hameed, B.H.; Abdulhameed, A.S. Synthesis of biohybrid magnetic chitosan-polyvinyl alcohol/MgO nanocomposite blend for Remazol brilliant blue R dye adsorption: Solo and collective parametric optimization. Polym. Bull. 2023, 80, 4927–4947. [Google Scholar] [CrossRef]
- Jebli, A.; Amri, A.E.; Hsissou, R.; Lebkiri, A.; Zarrik, B.; Bouhassane, F.Z.; Hbaiz, E.M.; Rifi, E.H.; Lebkiri, A. Synthesis of a chitosan@hydroxyapatite composite hybrid using a new approach for high-performance removal of Crystal violet dye in aqueous solution, equilibrium isotherms and process optimization. J. Taiwan Inst. Chem. Eng. 2023, 149, 105006. [Google Scholar] [CrossRef]
- Salahuddin, N.; El-Daly, H.; El Sharkawy, R.G.; Nasr, B.T. Synthesis and efficacy of PPy/CS/GO nanocomposites for adsorption of Ponceau 4R dye. Polymer 2018, 146, 291–303. [Google Scholar] [CrossRef]
- Türkeş, E.; Sağ Açıkel, Y. Synthesis and characterization of magnetic halloysite–chitosan nanocomposites: Use in the removal of Methylene blue in wastewaters. Int. J. Environ. Sci. Technol. 2020, 17, 1281–1294. [Google Scholar] [CrossRef]
- Pawariya, V.; De, S.; Dutta, J. Synthesis and characterization of a new developed modified-chitosan Schiff base with improved antibacterial properties for the removal of Bismarck brown R and Eosin Y dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2023, 6, 100352. [Google Scholar] [CrossRef]
- Rajendiran, R.; Patchaiyappan, A.; Harisingh, S.; Balla, P.; Paari, A.; Ponnala, B.; Perupogu, V.; Lassi, U.; Seelam, P.K. Synergistic effects of graphene oxide grafted chitosan & decorated MnO2 nanorods composite materials application in efficient removal of toxic industrial dyes. J. Water Process Eng. 2022, 47, 102704. [Google Scholar] [CrossRef]
- Doan, L.; Nguyen, T.M.D.; Le, T.M.; Huynh, K.G.; Quach, T.P.T. Surface modifications of superparamagnetic iron oxide nanoparticles with polyvinyl alcohol, chitosan, and activated carbon or graphite as Methylene blue adsorbents—Comparative study. Coatings 2023, 13, 1797. [Google Scholar] [CrossRef]
- Quach, T.P.T.; Doan, L. Surface Modifications of superparamagnetic iron oxide nanoparticles with polyvinyl alcohol, chitosan, and graphene oxide as Methylene blue adsorbents. Coatings 2023, 13, 1333. [Google Scholar] [CrossRef]
- Raval, N.P.; Priyadarshi, G.V.; Mukherjee, S.; Zala, H.; Fatma, D.; Bonilla-Petriciolet, A.; Abdelmottaleb, B.L.; Duclaux, L.; Trivedi, M.H. Statistical physics modeling and evaluation of adsorption properties of chitosan-zinc oxide nanocomposites for the removal of an anionic dye. J. Environ. Chem. Eng. 2022, 10, 108873. [Google Scholar] [CrossRef]
- Vardikar, H.S.; Bhanvase, B.A.; Rathod, A.P.; Sonawane, S.H. Sonochemical synthesis, characterization and sorption study of kaolin-chitosan-TiO2 ternary nanocomposite: Advantage over conventional method. Mater. Chem. Phys. 2018, 217, 457–467. [Google Scholar] [CrossRef]
- Jindal, R. Sodium alginate and chitosan based amphoteric nanocomposites modified with graphene oxide and bentonite as an efficient adsorbent for both anionic and cationic dyes. J. Polym. Environ. 2023, 31, 264–286. [Google Scholar] [CrossRef]
- Kandil, H.; Ali, H. Simultaneous removal of cationic Crystal violet and anionic Reactive yellow dyes using eco-friendly chitosan functionalized by talc and cloisite 30B. J. Polym. Environ. 2023, 31, 1456–1477. [Google Scholar] [CrossRef]
- Rastgordani, M.; Zolgharnein, J. Simultaneous determination and optimization of Titan yellow and Reactive blue 4 dyes removal using chitosan@hydroxyapatite nanocomposites. J. Polym. Environ. 2021, 29, 1789–1807. [Google Scholar] [CrossRef]
- Patel, S.R.; Patel, M.P. Selective capture of anionic and cationic dyes via chitosan-g-poly-(IA-co-DADMAC)/Fe3O4 polymer composite hydrogel. Polym. Bull. 2022, 79, 11079–11101. [Google Scholar] [CrossRef]
- Saharan, P.; Kumar, V.; Sharma, A.K.; Mahmud, H.N.M.E.; Mohamad, N.B.; Santos, J.H.; Zakaria, S.N.A. Scalable fabrication of chitosan-grafted silica bionanocomposite for the superb sequestration of anionic dye from aqueous solution. Emergent Mater. 2020, 3, 871–879. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Ayoup, M.S.; Omer, A.M.; Hammad, E.N.; Eltaweil, A.S. Sandwich-like construction of a new aminated chitosan Schiff base for efficient removal of Congo red. Appl. Water Sci. 2023, 13, 67. [Google Scholar] [CrossRef]
- Abou Alsoaud, M.M.; Taher, M.A.; Hamed, A.M.; Elnouby, M.S.; Omer, A.M. Reusable kaolin impregnated aminated chitosan composite beads for efficient removal of Congo red dye: Isotherms, kinetics and thermodynamics studies. Sci. Rep. 2022, 12, 12972. [Google Scholar] [CrossRef]
- Myneni, V.R.; Kanidarapu, N.R.; Shaik, F.; Vanalapati, M. Response Surface Modeling of the removal of Methyl orange dye from an aqueous solution using magnesium oxide nanoparticles immobilized on chitosan. Iran. J. Chem. Chem. Eng. 2022, 41, 1602–1618. [Google Scholar] [CrossRef]
- Abootorabi, Z.; Sohrabi, M.R.; Mortazavinik, S. Removing diazo Direct red 81 using chitosan/zero-valent iron nanocomposite from aqueous solutions and process optimization. Int. J. Environ. Anal. Chem. 2023, 103, 1168–1185. [Google Scholar] [CrossRef]
- Hasan, I.; Bhatia, D.; Walia, S.; Singh, P. Removal of Malachite green by polyacrylamide-g-chitosan γ-Fe2O3 nanocomposite-an application of central composite design. Groundw. Sustain. Dev. 2020, 11, 100378. [Google Scholar] [CrossRef]
- Blanco, L.; Martínez-Rico, O.; Domínguez, Á.; González, B. Removal of Acid blue 80 from aqueous solutions using chitosan-based beads modified with choline chloride:urea Deep Eutectic Solvent and FeO. Water Resour. Ind. 2023, 29, 100195. [Google Scholar] [CrossRef]
- Ali, H.E.; Nasef, S.M.; Gad, Y.H. Remediation of Astrazon blue and Lerui acid brilliant blue dyes from waste solutions using amphoteric superparamagnetic nanocomposite hydrogels based on chitosan prepared by gamma rays. Carbohydr. Polym. 2022, 283, 119149. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Jin, P.; Guo, W.; Liu, J.; Wang, W.; Li, P.; Cao, Y.; Zhang, L.; Zhang, Y. Recyclable magnetic iron immobilized onto chitosan with bridging Cu ion for the enhanced adsorption of Methyl orange. Molecules 2023, 28, 2307. [Google Scholar] [CrossRef]
- Jawad, A.H.; Rangabhashiyam, S.; Abdulhameed, A.S.; Syed-Hassan, S.S.A.; Alothman, Z.A.; Wilson, L.D. Process optimization and adsorptive mechanism for Reactive blue 19 dye by magnetic crosslinked chitosan/MgO/Fe3O4 biocomposite. J. Polym. Environ. 2022, 30, 2759–2773. [Google Scholar] [CrossRef]
- Huang, L.Y.; Li, W.; Du, N.; Lu, H.Q.; Meng, L.D.; Huang, K.Y.; Li, K. Preparation of quaternary ammonium magnetic chitosan microspheres and their application for Congo red adsorption. Carbohydr. Polym. 2022, 297, 119995. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, X.; Zhao, X.; Lou, T. Preparation of millimeter-scale hollow sphere with cationic chitosan/ dimethyl diallyl ammonium chloride /carboxymethyl cellulose terpolymer and its selective removal of anionic dye. J. Clean. Prod. 2022, 331, 130017. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, X. Preparation of chitosan-diatomite/calcium alginate composite hydrogel beads for the adsorption of Congo red dye. Water 2023, 15, 2254. [Google Scholar] [CrossRef]
- Liu, D.; Yuan, J.; Li, J.; Zhang, G. Preparation of chitosan poly(methacrylate) composites for adsorption of Bromocresol green. ACS Omega 2019, 4, 12680–12686. [Google Scholar] [CrossRef]
- Nga, N.K.; Thuy Chau, N.T.; Viet, P.H. Preparation and characterization of a chitosan/MgO composite for the effective removal of Reactive blue 19 dye from aqueous solution. J. Sci. Adv. Mater. Devices 2020, 5, 65–72. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Salama, A.; El-Ziaty, A.K.; Hassan, H. Preparation and adsorption properties of chitosan/silica/Fe3O4 nanocomposite. Cellul. Chem. Technol. 2020, 54, 601–608. [Google Scholar] [CrossRef]
- Saadat, A.; Banaei, A.; Sattarifar, M.; Pargolghasemi, P. Preparation 2-hydroxy-1-naphthaldehyde cross-linked Fe3O4@chitosan-polyacrylamide nanocomposite for removal of Everzol black from aqueous solutions. Sci. Rep. 2023, 13, 10618. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, C.; Zheng, H.; Wang, Y.; Zhao, C.; Zhao, C.; Zhang, S. Polymer-grafted magnetic microspheres for enhanced removal of Methylene blue from aqueous solutions. RSC Adv. 2017, 7, 47029–47037. [Google Scholar] [CrossRef]
- Alfuraydi, R.T.; Al-Harby, N.F.; Alminderej, F.M.; Elmehbad, N.Y.; Mohamed, N.A. Poly (vinyl alcohol) hydrogels boosted with cross-linked chitosan and silver nanoparticles for efficient adsorption of Congo red and Crystal violet dyes. Gels 2023, 9, 882. [Google Scholar] [CrossRef]
- Pérez-Calderón, J.; Scian, A.; Ducos, M.; Santos, V.; Zaritzky, N. Performance of oxalic acid-chitosan/alumina ceramic biocomposite for the adsorption of a reactive anionic azo dye. Environ. Sci. Pollut. Res. 2021, 28, 67032–67052. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Piri, F.; Zamani, A. One-pot synthesis of chitosan/iron oxide nanocomposite as an eco-friendly bioadsorbent for water remediation of Methylene blue. Micro Nano Lett. 2017, 12, 338–343. [Google Scholar] [CrossRef]
- Angela Mwesigye, K.; Zhou, B.; Wang, F.; Zhu, L.; Tang, Y. Novel dye removing agent based on CTS-g-P(AA-co-NIPAM)/GO composite. Arab. J. Chem. 2023, 16, 104581. [Google Scholar] [CrossRef]
- Ding, C.; Xue, M.; Zhang, Y.; Su, J.; Wang, H. Novel chitosan/GO@Fe3O4 porous microspheres with magnetic separation function for the removal of Congo red from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2023, 149, 105008. [Google Scholar] [CrossRef]
- Parlayıcı, Ş. Novel chitosan/citric acid modified pistachio shell/halloysite nanotubes cross-linked by glutaraldehyde biocomposite beads applied to Methylene blue removal. Int. J. Phytoremediation 2023, 26, 11–26. [Google Scholar] [CrossRef]
- Xu, G.; Zhu, Y.; Wang, X.; Wang, S.; Cheng, T.; Ping, R.; Cao, J.; Lv, K. Novel chitosan and Laponite based nanocomposite for fast removal of Cd(II), Methylene blue and Congo red from aqueous solution. E-Polym. 2019, 19, 244–256. [Google Scholar] [CrossRef]
- Pereira, M.B.B.; Honório, L.M.C.; Lima-Júnior, C.G.; Silva Filho, E.C.; Gaslain, F.; Rigaud, B.; Fonseca, M.G.; Jaber, M. Modulating the structure of organofunctionalized hydroxyapatite/tripolyphosphate/chitosan spheres for dye removal. J. Environ. Chem. Eng. 2020, 8, 103980. [Google Scholar] [CrossRef]
- Priyadarshi, G.; Raval, N.P.; Trivedi, M.H. Microwave-assisted synthesis of cross-linked chitosan-metal oxide nanocomposite for methyl orange dye removal from unary and complex effluent matrices. Int. J. Biol. Macromol. 2022, 219, 53–67. [Google Scholar] [CrossRef]
- Abdul Mubarak, N.S.; Bahrudin, N.N.; Jawad, A.H.; Hameed, B.H.; Sabar, S. Microwave enhanced synthesis of sulfonated chitosan-montmorillonite for effective removal of Methylene blue. J. Polym. Environ. 2021, 29, 4027–4039. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Bai, X.; Xu, J.; Zhang, J.; Zhang, G.; Huang, C.; Liu, W.; Huang, C.; Xiong, X. Microfluidic preparation of magnetic chitosan microsphere and its adsorption towards Congo red. J. Polym. Res. 2023, 30, 77. [Google Scholar] [CrossRef]
- Ramezani, S.; Zahedi, P.; Bahrami, S.H.; Nemati, Y. Microfluidic fabrication of nanoparticles based on ethyl acrylate-functionalized chitosan for adsorption of Methylene blue from aqueous solutions. J. Polym. Environ. 2019, 27, 1653–1665. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Ray, S.K. Micro- and nano-sized bentonite filled composite superabsorbents of chitosan and acrylic copolymer for removal of synthetic dyes from water. Appl. Clay Sci. 2014, 101, 510–520. [Google Scholar] [CrossRef]
- Dolatkhah, A.; Wilson, L.D. Magnetite/polymer brush nanocomposites with switchable uptake behavior toward Methylene blue. ACS Appl. Mater. Interfaces 2016, 8, 5595–5607. [Google Scholar] [CrossRef] [PubMed]
- Babacan, T.; Doğan, D.; Erdem, Ü.; Metin, A.Ü. Magnetically responsive chitosan-based nanoparticles for remediation of anionic dyes: Adsorption and magnetically triggered desorption. Mater. Chem. Phys. 2022, 284, 126032. [Google Scholar] [CrossRef]
- Freire, T.M.; Fechine, L.M.U.D.; Queiroz, D.C.; Freire, R.M.; Denardin, J.C.; Ricardo, N.M.P.S.; Rodrigues, T.N.B.; Gondim, D.R.; Junior, I.J.S.; Fechine, P.B.A. Magnetic porous controlled Fe3O4–chitosan nanostructure: An ecofriendly adsorbent for efficient removal of azo dyes. Nanomaterials 2020, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Mirzai, M.; Asadabadi, S. Magnetic nanocomposites containing low and medium-molecular weight chitosan for dye Adsorption: Hydrophilic property versus functional groups. J. Polym. Environ. 2022, 30, 1560–1573. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Y.; Du, Q.; Chen, B.; Chen, K.; Zhang, Y.; Wang, M.; Sun, Y.; Zhao, S.; Jing, Z.; et al. Efficient adsorption of Congo red by MIL-53(Fe)/chitosan composite hydrogel spheres. Microporous Mesoporous Mater. 2023, 348, 112404. [Google Scholar] [CrossRef]
- Kafil, M.; Nasab, S.B.; Moazed, H.; Jokiniemi, J.; Lähde, A.; Bhatnagar, A. Efficient removal of azo dyes from water with chitosan/carbon nanofloas a novel nanocomposite synthesized by pyrolysis technique. Desalin. Water Treat. 2019, 142, 308–320. [Google Scholar] [CrossRef]
- Kaur, K.; Vaid, V.; Jindal, R. Efficient removal of Rose Bengal and Malachite green dyes using green and sustainable chitosan/CMC/bentonite-based hydrogel materials. Polym. Bull. 2023, 80, 6609–6634. [Google Scholar] [CrossRef]
- Barus, D.A.; Humaidi, S.; Ginting, R.T.; Sitepu, J. Enhanced adsorption performance of chitosan/cellulose nanofiber isolated from durian peel waste/graphene oxide nanocomposite hydrogels. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100650. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Çetinkaya, S.; Yenidünya, A.F.; Başoğlu-Ünal, F.; Ece, A. Epichlorohydrin and tripolyphosphate-crosslinked chitosan–kaolin composite for Auramine O dye removal from aqueous solutions: Experimental study and DFT calculations. Int. J. Biol. Macromol. 2022, 199, 318–330. [Google Scholar] [CrossRef]
- Kumar, A.; Jeyabalan, J.; Priyan, V.V.; Charan Patra, C.; Narayanasamy, S. Fabrication of a novel bio-polymer adsorbent with high adsorptive capacity towards organic dyes. Ind. Crops Prod. 2023, 203, 117166. [Google Scholar] [CrossRef]
- Parshi, N.; Pan, D.; Dhavle, V.; Jana, B.; Maity, S.; Ganguly, J. Fabrication of lightweight and reusable salicylaldehyde functionalized chitosan as adsorbent for dye removal and its mechanism. Int. J. Biol. Macromol. 2019, 141, 626–635. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- P, H.R. Kapillarchemie, eine darstellung der chemie der kolloide und verwandter gebiete. Nature 1911, 85, 534–535. [Google Scholar] [CrossRef]
- Temkin, M.; Pyzhev, V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Physicochim. 1940, 12, 327. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the characteristic curve of activated charcoal. Proc. Acad. Sciences. Phys. Chem. Sect. USSR 1947, 55, 331–333. [Google Scholar]
- Toth, J. State equation of the solid-gas interface layers. Acta Chim. Hung. 1971, 69, 311–328. [Google Scholar]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Liu, D.M.; Dong, C.; Zhong, J.; Ren, S.; Chen, Y.; Qiu, T. Facile preparation of chitosan modified magnetic kaolin by one-pot coprecipitation method for efficient removal of Methyl orange. Carbohydr. Polym. 2020, 245, 116572. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Zhao, X.; Zhang, Y. Fabrication of three-dimensional porous β-cyclodextrin/chitosan functionalized graphene oxide hydrogel for Methylene blue removal from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 1–10. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Zhang, D.; Peng, H.; Ren, Y. Facile preparation of taurine modified magnetic chitosan nanocomposites as biodegradable adsorbents toward Methylene blue. Environ. Technol. 2021, 42, 3191–3204. [Google Scholar] [CrossRef]
- Kumari, N.; Behera, M.; Singh, R. Facile synthesis of biopolymer decorated magnetic coreshells for enhanced removal of xenobiotic azo dyes through experimental modelling. Food Chem. Toxicol. 2023, 171, 113518. [Google Scholar] [CrossRef]
- Malek, N.N.A.; Jawad, A.H.; Ismail, K.; Razuan, R.; Alothman, Z.A. Fly ash modified magnetic chitosan-polyvinyl alcohol blend for Reactive orange 16 dye removal: Adsorption parametric optimization. Int. J. Biol. Macromol. 2021, 189, 464–476. [Google Scholar] [CrossRef]
- Jamali, M.; Akbari, A. Facile fabrication of magnetic chitosan hydrogel beads and modified by interfacial polymerization method and study of adsorption of cationic/anionic dyes from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 105175. [Google Scholar] [CrossRef]
- Gul, K.; Sohni, S.; Waqar, M.; Ahmad, F.; Norulaini, N.A.N.; AK, M.O. Functionalization of magnetic chitosan with graphene oxide for removal of cationic and anionic dyes from aqueous solution. Carbohydr. Polym. 2016, 152, 520–531. [Google Scholar] [CrossRef]
- Zango, Z.U.; Dennis, J.O.; Aljameel, A.I.; Usman, F.; Ali, M.K.M.; Abdulkadir, B.A.; Algessair, S.; Aldaghri, O.A.; Ibnaouf, K.H. Effective removal of Methylene blue from simulated wastewater using ZnO-chitosan nanocomposites: Optimization, kinetics, and isotherm studies. Molecules 2022, 27, 4746. [Google Scholar] [CrossRef]
- Jahanbakhsh, Z.; Hosseinzadeh, H.; Massoumi, B. Fabrication of magnetic β-CD/chitosan nanocomposite as an efficient and recyclable dye adsorbent. Polym.-Plast. Technol. Mater. 2020, 59, 1932–1943. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Gas and solution uptake properties of graphene oxide-based composite materials: Organic vs. inorganic cross-linkers. J. Compos. Sci. 2019, 3, 80. [Google Scholar] [CrossRef]
- Tamer, Y.; Koşucu, A.; Berber, H. Graphene oxide incorporated chitosan/acrylamide/itaconic acid semi-interpenetrating network hydrogel bio-adsorbents for highly efficient and selective removal of cationic dyes. Int. J. Biol. Macromol. 2022, 219, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 2, 15. [Google Scholar]
- Alzahrani, H.K.; Katowah, D.F. Fabrication of network nanocomposite of polyaniline coating chitosan-graphene oxide-functionalized carbon nanotube and its efficacy in removing dyes from aqueous solution. Nanocomposites 2023, 9, 183–202. [Google Scholar] [CrossRef]






| Dye Class | Examples | |
|---|---|---|
| Azo dyes |  |  |
| Congo red | Methyl orange | |
| Anthraquinone dyes |  |  |
| Alizarin | Rhodamine B | |
| Indigo dyes |  |  |
| Indigo carmine | Thioindigo | |
| Xanthene dyes |  |  |
| Eosin | Rose Bengal | |
| Phthalocyanine dyes |  |  |
| Direct blue 199 | Phthalocyanine green | |
| Nitro dyes |  |  |
| Martius yellow | Naphtol yellow | |
| Nitroso dyes |  |  |
| 2-nitroso-1-naphtol | Fast green O | |
| Arylmethane dyes |  |  |
| Malachite green | Crystal violet | |
| Dye Class | Examples | |
|---|---|---|
| Acid dyes (anionic dyes) |  |  |
| Sunset yellow | Allura red | |
| Basic dyes (cationic dyes) |  |  |
| Methylene blue | Eriochrome black T | |
| Reactive dyes |  |  |
| Reactive red 1 | Reactive blue 19 | |
| Direct dyes |  |  |
| Direct yellow 44 | Direct green 6 | |
| Vat dyes |  |  |
| Vat violet 1 | Vat orange 1 | |
| Sulfur dyes |  |  |
| Sulfur blue | Sulfur brilliant green | |
| Disperse dyes |  |  |
| Disperse red 60 | Disperse blue 72 | |
| Equilibrium Isotherm | Equation | Parameters’ Significance * |
|---|---|---|
| Two-terms isotherms | ||
| Langmuir | QmL—maximum Langmuir uptake, mg/g KL—Langmuir constant, L/mg | |
| Extended Langmuir | QmL—maximum Langmuir uptake for component, mg/g KL—Langmuir constant for component, L/mg | |
| Modified Langmuir | QmL—maximum Langmuir uptake for component, mg/g KL—Langmuir constant for component, L/mg η—additional interaction factor | |
| Freundlich | KF—Freundlich constant, (mg/g)(L/mg)1/n nF—Freundlich constant, dimensionless | |
| Extended Freundlich | KF—Freundlich constant for component, L/mg n —Adsorption intensity for components in a solution xi, xj—Experimental constant values of plot Qe,i vs. Ce,i, dimensionless yi, yj—Experimental constant values of plot Qe,j vs. Ce,j, dimensionless zi, zj—Experimental constant values of plot Qe,i vs. Ce,i, dimensionless | |
| Langmuir–Freundlich | QmLF—maximum Langmuir–Freundlich uptake, mg/g KLF—Langmuir–Freundlich constant, L/g n—heterogeneity index, dimensionless | |
| Modified Langmuir–Freundlich | QmLF—maximum Langmuir–Freundlich uptake, mg/g ka—Affinity coefficient for adsorption, L/mg | |
| Temkin | R—gas constant, R = 8.314 J/(mol K) T—temperature, K KT—Temkin constant, L/mg bT—Temkin constant, J/mg | |
| Hill | QSH—Hill maximum uptake saturation, mg/L Kd—Hill constant nH—Hill cooperativity coefficient of the binding interaction | |
| Flory— Huggins | QmFH—Flory–Huggins maximum adsorption capacity, mg/g KFH—Flory–Huggins equilibrium constant, L/mg n—exponent, dimensionless | |
| Dubinin– Radushkevich | Qs—Theoretical monolayer saturation capacity, mg/g B—Constant of the sorption energy, mol2/kJ2 ε—Polanyi potential, dimensionless | |
| Elovich | QmE—Elovich maximum adsorption capacity, mg/g KFG—Elovich equilibrium constant, L/mg | |
| Liu | QmLi—Liu maximum uptake, mg/g Kg—Liu constant, L/g nL—heterogeneity index, dimensionless | |
| Fowler– Guggenheim | QFG—Fowler–Guggenheim maximum adsorption capacity, mg/g KFG—Fowler–Guggenheim equilibrium constant, L/mg R—gas constant, R = 8.314 J/(mol K) T—temperature (K) ω—interaction energy between adsorbed molecules, Kj/mol | |
| Harkin–Jura | A—Harkin–Jura constant, dimensionless B—Harkin–Jura constant, dimensionless | |
| Three-terms isotherms | ||
| Toth | QTo—Toth maximum uptake, mg/g KTo—Toth constant, L/mg nTo—Toth constant, dimensionless | |
| Sips | QS—Sips maximum uptake, mg/g KS—Sips constant, L/mg nS—Sips constant, dimensionless | |
| Redlich– Peterson | KR—Redlich–Peterson constant, L/g aR—Redlich–Peterson constant, 1/mg bg—Redlich–Peterson exponent, dimensionless | |
| Radke–Prausnitz | QMRP—Radke–Prausnitz maximum adsorption capacity, mg/g KRP—Radke–Prausnitz equilibrium constant, dimensionless MRP—Radke–Prausnitz exponent, dimensionless | |
| Kinetic Model | Equation | Parameters Significance * |
|---|---|---|
| Pseudo-1st-order | k1—pseudo-first-order constant rate, min−1 | |
| Pseudo-2nd-order | k2—pseudo-second-order constant rate, g/(mg·min) | |
| Mixed 1,2-order | k1—pseudo-first-order constant rate, min−1 k2—pseudo-second-order constant rate, g/(mg·min) | |
| Pseudo-nth-order | kn—pseudo-nth-order constant rate | |
| Fractal-like pseudo-1st-order | k′1—fractal-like pseudo-first-order constant rate, min−(1-h) h—fractal exponent, 0 ≤ h ≤ 1, dimensionless | |
| Fractal-like pseudo-2nd-order | k2—fractal-like pseudo-second-order constant rate, g mg−1 min−(1-h)) | |
| Fractal-like mixed 1,2 order | K′1—fractal-like pseudo-first-order constant rate, min−(1-h) h—fractal exponent, 0 ≤ h ≤ 1, dimensionless | |
| Intraparticle diffusion | kp—rate constant of the intraparticle diffusion kinetic model, mg/g min1/2 c—constant, dimensionless | |
| Elovich | β—extent of surface coverage and activation energy for chemisorption, g/mg α—initial adsorption rate, mg/(g·min) | |
| Mixed surface reaction—diffusion controlled kinetic | C0—initial concentration solute, mg/L a—model coefficient, mg/L b—model coefficient, 1/min weq—relative equilibrium uptake | |
| Liquid film diffusion | KFD—liquid film diffusion constant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigoraș, C.-G.; Simion, A.-I.; Drob, C. Hydrogels Based on Chitosan and Nanoparticles and Their Suitability for Dyes Adsorption from Aqueous Media: Assessment of the Last-Decade Progresses. Gels 2024, 10, 211. https://doi.org/10.3390/gels10030211
Grigoraș C-G, Simion A-I, Drob C. Hydrogels Based on Chitosan and Nanoparticles and Their Suitability for Dyes Adsorption from Aqueous Media: Assessment of the Last-Decade Progresses. Gels. 2024; 10(3):211. https://doi.org/10.3390/gels10030211
Chicago/Turabian StyleGrigoraș, Cristina-Gabriela, Andrei-Ionuț Simion, and Cătălin Drob. 2024. "Hydrogels Based on Chitosan and Nanoparticles and Their Suitability for Dyes Adsorption from Aqueous Media: Assessment of the Last-Decade Progresses" Gels 10, no. 3: 211. https://doi.org/10.3390/gels10030211
APA StyleGrigoraș, C. -G., Simion, A. -I., & Drob, C. (2024). Hydrogels Based on Chitosan and Nanoparticles and Their Suitability for Dyes Adsorption from Aqueous Media: Assessment of the Last-Decade Progresses. Gels, 10(3), 211. https://doi.org/10.3390/gels10030211