Evaluation of the Swelling Properties and Sorption Capacity of Maltodextrin-Based Cross-Linked Polymers
Abstract
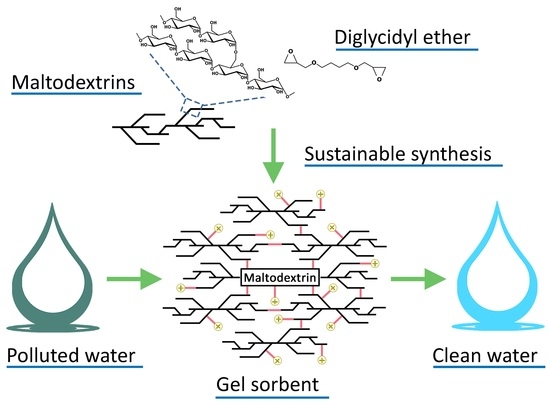
:1. Introduction
2. Results and Discussion
2.1. Material Design and Characterization
2.2. Water Absorption Capacity
2.3. Cross-Linking Density Determination Using the Flory–Rehner Theory
2.4. Rheological Measurements
2.5. Evaluation of Sorption Performances
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. BDE Cross-Linked Polymer (GLU_BDE)
4.3. TTE Cross-Linked Polymer (GLU_TTE)
4.4. Scanning Electron Microscopy (SEM)
4.5. Thermal Analyses
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Powder X-ray Diffraction
4.8. Elemental Analysis
4.9. Zeta Potential
4.10. Water Absorption Capacity (WAC)
4.11. Cross-Linking Density Determination Using Swelling Experiments
4.12. Rheological Measurements
4.13. Sorption Tests
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pycia, K.; Juszczak, L.; Gałkowska, D.; Socha, R.; Jaworska, G. Maltodextrins from Chemically Modified Starches. Production and Characteristics. Starch-Stärke 2017, 69, 1600199. [Google Scholar] [CrossRef]
- Anceschi, A.; Magnacca, G.; Trotta, F.; Zanetti, M. Preparation and Characterization of Microporous Carbon Spheres from High Amylose Pea Maltodextrin. RSC Adv. 2017, 7, 36117–36123. [Google Scholar] [CrossRef]
- Lumdubwong, N.; Seib, P.A. Low- and Medium-DE Maltodextrins From Waxy Wheat Starch: Preparation and Properties. Starch-Stärke 2001, 53, 605. [Google Scholar] [CrossRef]
- Ananya, K.V.; Preethi, S.; Patil, A.B.; Gowda, D.V. Recent Review on Nano Sponge. Int. J. Res. Pharm. Sci. 2020, 11, 1085–1096. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking Biopolymers for Biomedical Applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- O’Connor, N.A.; Abugharbieh, A.; Yasmeen, F.; Buabeng, E.; Mathew, S.; Samaroo, D.; Cheng, H.-P. The Crosslinking of Polysaccharides with Polyamines and Dextran-Polyallylamine Antibacterial Hydrogels. Int. J. Biol. Macromol. 2015, 72, 88–93. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Parra-Ruiz, F.J.; Casado, A.L.; Román, J.S. Thermal Crosslinking of Maltodextrin and Citric Acid. Methodology to Control Polycondensation Reaction under Processing Conditions. Polym. Polym. Compos. 2016, 24, 643–654. [Google Scholar] [CrossRef]
- Gharakhloo, M.; Sadjadi, S.; Rezaeetabar, M.; Askari, F.; Rahimi, A. Cyclodextrin-Based Nanosponges for Improving Solubility and Sustainable Release of Curcumin. ChemistrySelect 2020, 5, 1734–1738. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-Based Nanosponges: A Critical Review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid Removal of Organic Micropollutants from Water by a Porous β-Cyclodextrin Polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically Cross-Linked and Grafted Cyclodextrin Hydrogels: From Nanostructures to Drug-Eluting Medical Devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and Applications of Adsorbents Containing Cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Nobuhiko, Y.; Jun, N.; Teruo, O.; Yasuhisa, S. Regulated Release of Drug Microspheres from Inflammation Responsive Degradable Matrices of Crosslinked Hyaluronic Acid. J. Control. Release 1993, 25, 133–143. [Google Scholar] [CrossRef]
- Komiyama, M.; Hirai, H. Preparation of Immobilized β-Cyclodextrins by Use of Alkanediol Diglycidyl Ethers as Crosslinking Agents and Their Guest Binding Abilities. Polym. J. 1987, 19, 773–775. [Google Scholar] [CrossRef]
- Huang, L.L.H.; Lee, P.C.; Chen, L.W.; Hsieh, K.H. Comparison of Epoxides on Grafting Collagen to Polyurethane and Their Effects on Cellular Growth. J. Biomed. Mater. Res. 1998, 39, 630–636. [Google Scholar] [CrossRef]
- Suye, S.; Mizusawa, A. Cross-Linking of Chitosan Membrane with Polyethylene Glycol Diglycidyl Ether for Immobilization of Uricase. Sen’i Gakkaishi 1999, 55, 73–77. [Google Scholar] [CrossRef]
- Rodriguez-Tenreiro, C.; Alvarez-Lorenzo, C.; Rodriguez-Perez, A.; Concheiro, A.; Torres-Labandeira, J.J. New Cyclodextrin Hydrogels Cross-Linked with Diglycidylethers with a High Drug Loading and Controlled Release Ability. Pharm. Res. 2006, 23, 121–130. [Google Scholar] [CrossRef]
- Martucci, J.F.; Espinosa, J.P.; Ruseckaite, R.A. Physicochemical Properties of Films Based on Bovine Gelatin Cross-Linked with 1,4-Butanediol Diglycidyl Ether. Food Bioprocess Technol. 2015, 8, 1645–1656. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Zhang, J.; Bai, Y.; Gao, Q.; Li, J.; Li, L. A New Flexible Soy-Based Adhesive Enhanced with Neopentyl Glycol Diglycidyl Ether: Properties and Application. Polymers 2016, 8, 346. [Google Scholar] [CrossRef]
- Roig, A.; Hidalgo, P.; Ramis, X.; De La Flor, S.; Serra, À. Vitrimeric Epoxy-Amine Polyimine Networks Based on a Renewable Vanillin Derivative. ACS Appl. Polym. Mater. 2022, 4, 9341–9350. [Google Scholar] [CrossRef]
- Hou, N.; Wang, R.; Wang, F.; Bai, J.; Zhou, J.; Zhang, L.; Hu, J.; Liu, S.; Jiao, T. Fabrication of Hydrogels via Host-Guest Polymers as Highly Efficient Organic Dye Adsorbents for Wastewater Treatment. ACS Omega 2020, 5, 5470–5479. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel Adsorbents for the Removal of Hazardous Pollutants—Requirements and Available Functions as Adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Cecone, C.; Iudici, M.; Ginepro, M.; Zanetti, M.; Trotta, F.; Bracco, P. Dextrin-Based Adsorbents Synthesized via a Sustainable Approach for the Removal of Salicylic Acid from Water. Nanomaterials 2023, 13, 2805. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel Applications for Adsorption of Contaminants in Water and Wastewater Treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Goh, S.S.; Liow, S.S.; Xue, K.; Loh, X.J. Molecular Gel Sorbent Materials for Environmental Remediation and Wastewater Treatment. J. Mater. Chem. A Mater. 2019, 7, 18759–18791. [Google Scholar] [CrossRef]
- Cecone, C.; Costamagna, G.; Ginepro, M.; Trotta, F. One-Step Sustainable Synthesis of Cationic High- Swelling Polymers Obtained from Starch-Derived Maltodextrins. RSC Adv. 2021, 11, 7653–7662. [Google Scholar] [CrossRef]
- Smith, V.H. Eutrophication of Freshwater and Coastal Marine Ecosystems: A Global Problem. Environ. Sci. Pollut. Res. 2003, 10, 126–139. [Google Scholar] [CrossRef]
- Morse, G.K.; Brett, S.W.; Guy, J.A.; Lester, J.N. Review: Phosphorus Removal and Recovery Technologies. Sci. Total Environ. 1998, 212, 69–81. [Google Scholar] [CrossRef]
- Azam, H.M.; Alam, S.T.; Hasan, M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the Environment: Characteristics with Distribution and Effects, Removal Mechanisms, Treatment Technologies, and Factors Affecting Recovery as Minerals in Natural and Engineered Systems. Environ. Sci. Pollut. Res. 2019, 26, 20183–20207. [Google Scholar] [CrossRef] [PubMed]
- Gakstatter, J.H.; Bartsch, A.F.; Callahan, C.A. The Impact of Broadly Applied Effluent Phosphorus Standards on Eutrophication Control. Water Resour. Res. 1978, 14, 1155–1158. [Google Scholar] [CrossRef]
- Correll, D.L. The Role of Phosphorus in the Eutrophication of Receiving Waters: A Review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef]
- Costamagna, G.; Chiabrando, V.; Fassone, E.; Mania, I.; Gorra, R.; Ginepro, M.; Giacalone, G. Characterization and Use of Absorbent Materials as Slow-Release Fertilizers for Growing Strawberry: Preliminary Results. Sustainability 2020, 12, 6854. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint Pollution of Surface Waters with Phosphorus and Nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- De La Torre, M.L.; Grande, J.A.; Graiño, J.; Gómez, T.; Cerón, J.C. Characterization of AMD Pollution in the River Tinto (SW Spain). Geochemical Comparison between Generating Source and Receiving Environment. Water Air Soil Pollut. 2011, 216, 3–19. [Google Scholar] [CrossRef]
- Peplow, D. Environmental Impacts of Mining in Eastern Washington; University of Washington Water Center: Seattle, WA, USA, 1999. [Google Scholar]
- Călinescu, O.; Marin, N.M.; Ioniță, D.; Pascu, L.F.; Tudorache, A.; Surpățeanue, G.; Badea, I.A.; Aboul-Enein, H.Y. Selective Removal of Sulfate Ion from Different Drinking Waters. Environ. Nanotechnol. Monit. Manag. 2016, 6, 164–168. [Google Scholar] [CrossRef]
- Bowell, R.J.; Dill, S.; Cowan, J.; Wood, A. A Review of Sulfate Removal Options for Mine Waters. IMWA Proc. 1998, 1998, 329–342. [Google Scholar]
- Costamagna, G.; Volpi, G.; Ghibaudi, E.; Ginepro, M. Quantitative Insights on the Interaction between Metal Ions and Water Kefir Grains: Kinetics Studies and EPR Investigations. Nat. Prod. Res. 2020, 36, 3440–3444. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Ginepro, M.; Tafur-Marinos, J.; Zelano, V. Pollution Abatement of Heavy Metals in Different Conditions by Water Kefir Grains as a Protective Tool against Toxicity. J. Chem. 2019, 2019, 8763902. [Google Scholar] [CrossRef]
- Geurts, J.J.M.; Sarneel, J.M.; Willers, B.J.C.; Roelofs, J.G.M.; Verhoeven, J.T.A.; Lamers, L.P.M. Interacting Effects of Sulphate Pollution, Sulphide Toxicity and Eutrophication on Vegetation Development in Fens: A Mesocosm Experiment. Environ. Pollut. 2009, 157, 2072–2081. [Google Scholar] [CrossRef]
- Baldwin, D.S.; Mitchell, A. Impact of Sulfate Pollution on Anaerobic Biogeochemical Cycles in a Wetland Sediment. Water Res. 2012, 46, 965–974. [Google Scholar] [CrossRef]
- Archna; Sharma, S. K.; Sobti, R.C. Nitrate Removal from Ground Water: A Review. J. Chem. 2012, 9, 1667–1675. [Google Scholar] [CrossRef]
- Chopra, A.K.; Kumar Sharma, A.; Kumar, V. Overview of Electrolytic Treatment: An Alternative Technology for Purification of Wastewater. Arch. Appl. Sci. Res. 2011, 3, 191–206. [Google Scholar]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Musa, C.; Kervoëlen, A.; Danjou, P.-E.; Bourmaud, A.; Delattre, F. Bio-Based Unidirectional Composite Made of Flax Fibre and Isosorbide-Based Epoxy Resin. Mater. Lett. 2020, 258, 126818. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Jiang, Y.; Fan, L.; Feng, J.; Zhu, J. Synthesis and Properties of Phosphorus-Containing Bio-Based Epoxy Resin from Itaconic Acid. Sci. China Chem. 2014, 57, 379–388. [Google Scholar] [CrossRef]
- Hu, F.; La Scala, J.J.; Sadler, J.M.; Palmese, G.R. Synthesis and Characterization of Thermosetting Furan-Based Epoxy Systems. Macromolecules 2014, 47, 3332–3342. [Google Scholar] [CrossRef]
- Álvarez-Chávez, C.R.; Edwards, S.; Moure-Eraso, R.; Geiser, K. Sustainability of Bio-Based Plastics: General Comparative Analysis and Recommendations for Improvement. J. Clean. Prod. 2012, 23, 47–56. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Miao, S.; Chen, L.; Li, L.; Liang, Y. Understanding the Effect of Freeze-Drying on Microstructures of Starch Hydrogels. Food Hydrocoll. 2020, 101, 105509. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic Implication for Cross-Linking in Sterculia-Based Hydrogels and Their Use in GIT Drug Delivery. Biomacromolecules 2009, 10, 2515–2532. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A Comprehensive Review of Phosphorus Recovery from Wastewater by Crystallization Processes. Chemosphere 2018, 197, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Hoti, G.; Caldera, F.; Cecone, C.; Pedrazzo, A.R.; Anceschi, A.; Appleton, S.L.; Monfared, Y.K.; Trotta, F. Effect of the Cross-Linking Density on the Swelling and Rheological Behavior of Ester-Bridged β-Cyclodextrin Nanosponges. Materials 2021, 14, 478. [Google Scholar] [CrossRef] [PubMed]











| Test Performed | Sorbent | |
|---|---|---|
| GLU_BDE | GLU_TTE | |
| Tonset (°C) | 271 | 264 |
| N wt.% | 0.8 | 1.1 |
| Zeta potential (mV) | 10.4 ± 1.2 | 13.8 ± 1.7 |
| WAC (%) | 1293 ± 13 | 755 ± 5 |
| WAC (%) double swelling | 1560 ± 46 | 878 ± 56 |
| WAC (%) after NO3− sorption | 1433 ± 66 | / |
| WAC (%) after SO42− sorption | 1251 ± 173 | / |
| WAC (%) after PO43− sorption | 1215 ± 170 | / |
| G′ (Pa) | 2435 | 1285 |
| G″ (Pa) | 899 | 691 |
| υFR (mol/cm3) | 1.72 × 10−5 ± 6.55 × 10−7 | 5.64 × 10−5 ± 1.26 × 10−6 |
| υeR (mol/cm3) | 2.38 × 10−6 ± 9.68 × 10−7 | 2.83 × 10−6 ± 1.86 × 10−6 |
| Mc (g/mol) | 58,212 ± 2322 | 17,724 ± 385 |
| Sor(%) NO3−, 100 mg/L | 62.9 ± 3.1 | 79.7 ± 4.0 |
| Sor(mg/g) NO3−, 100 mg/L | 6.3 ± 3.1 | 8.0 ± 4.0 |
| Sor(%) NO3−, 200 mg/L | 54.7 ± 2.2 | 65.9 ± 2.6 |
| Sor(mg/g) NO3−, 200 mg/L | 10.9 ± 2.2 | 13.2 ± 2.6 |
| Sor(%) NO3−, 300 mg/L | 41.1 ± 1.4 | 57.4 ± 1.9 |
| Sor(mg/g) NO3−, 300 mg/L | 12.3 ± 1.4 | 17.2 ± 1.9 |
| Sor(%) NO3−, 400 mg/L | 29.0 ± 0.7 | 48.6 ± 1.2 |
| Sor(mg/g) NO3−, 400 mg/L | 11.6 ± 0.7 | 19.4 ± 1.2 |
| Sor(%) NO3−, 500 mg/L | 21.4 ± 0.4 | 43.2 ± 0.9 |
| Sor(mg/g) NO3−, 500 mg/L | 10.7 ± 0.4 | 21.6 ± 0.9 |
| Sor(%) NO3−, 1000 mg/L | 18.0 ± 0.3 | 38.0 ± 0.6 |
| Sor(mg/g) NO3−, 1000 mg/L | 18.0 ± 0.3 | 38.0 ± 0.6 |
| Sor(%) SO42−, 100 mg/L | 64.2 ± 3.2 | 94.6 ± 4.7 |
| Sor(mg/g) SO42−, 100 mg/L | 6.4 ± 3.2 | 9.4 ± 4.7 |
| Sor(%)SO42−, 200 mg/L | 51.0 ± 2.0 | 89.9 ± 3.6 |
| Sor(mg/g) SO42−, 200 mg/L | 10.2 ± 2.0 | 18.0 ± 3.6 |
| Sor(%) SO42−, 300 mg/L | 41.6 ± 1.4 | 77.2 ± 2.6 |
| Sor(mg/g) SO42−, 300 mg/L | 12.5 ± 1.4 | 23.2 ± 2.6 |
| Sor(%) SO42−, 400 mg/L | 31.8 ± 0.8 | 61.4 ± 1.5 |
| Sor(mg/g) SO42−, 400 mg/L | 12.7 ± 0.8 | 24.6 ± 1.5 |
| Sor(%) SO42−, 500 mg/L | 25.0 ± 0.5 | 51.6 ± 1.0 |
| Sor(mg/g) SO42−, 500 mg/L | 12.5 ± 0.5 | 25.8 ± 1.0 |
| Sor(%) SO42−, 1000 mg/L | 15.4 ± 0.3 | 29.4 ± 0.5 |
| Sor(mg/g) SO42−, 1000 mg/L | 15.4 ± 0.3 | 29.4 ± 0.5 |
| Sor(%) PO43−, pH 11.88 | 51.2 ± 1.6 | 93.8 ± 2.4 |
| Sor(mg/g) PO43−, pH 11.88 | 16.7 ± 1.6 | 30.6 ± 2.4 |
| Sor(%) PO43−, pH 8.49 | 83.5 ± 1.3 | 99.0 ± 0.2 |
| Sor(mg/g) PO43−, pH 8.49 | 26.9 ± 1.3 | 31.9 ± 0.2 |
| Sor(%) PO43−, pH 7.04 | 84.6 ± 3.2 | 91.1 ± 3.3 |
| Sor(mg/g) PO43−, pH 7.04 | 27.0 ± 3.2 | 29.1 ± 3.3 |
| Sor(%) PO43−, pH 5.25 | 79.5 ± 4.1 | 92.2 ± 2.9 |
| Sor(mg/g) PO43−, pH 5.25 | 25.5 ± 4.1 | 29.6 ± 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecone, C.; Hoti, G.; Caldera, F.; Ginepro, M.; Matencio, A.; Trotta, F. Evaluation of the Swelling Properties and Sorption Capacity of Maltodextrin-Based Cross-Linked Polymers. Gels 2024, 10, 232. https://doi.org/10.3390/gels10040232
Cecone C, Hoti G, Caldera F, Ginepro M, Matencio A, Trotta F. Evaluation of the Swelling Properties and Sorption Capacity of Maltodextrin-Based Cross-Linked Polymers. Gels. 2024; 10(4):232. https://doi.org/10.3390/gels10040232
Chicago/Turabian StyleCecone, Claudio, Gjylije Hoti, Fabrizio Caldera, Marco Ginepro, Adrián Matencio, and Francesco Trotta. 2024. "Evaluation of the Swelling Properties and Sorption Capacity of Maltodextrin-Based Cross-Linked Polymers" Gels 10, no. 4: 232. https://doi.org/10.3390/gels10040232
APA StyleCecone, C., Hoti, G., Caldera, F., Ginepro, M., Matencio, A., & Trotta, F. (2024). Evaluation of the Swelling Properties and Sorption Capacity of Maltodextrin-Based Cross-Linked Polymers. Gels, 10(4), 232. https://doi.org/10.3390/gels10040232