Enhancing the Quality of Low-Salt Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Using Psyllium Husk Powder: An Orthogonal Experimental Approach
Abstract
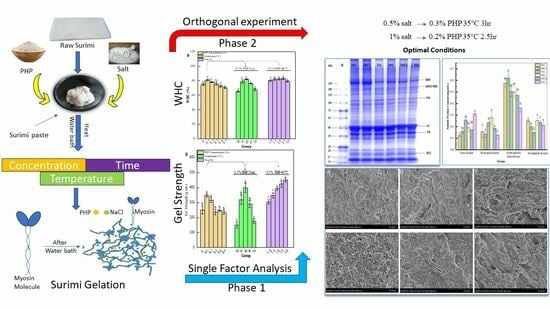
:1. Introduction
2. Results and Discussions
2.1. Single-Factor Test
2.1.1. Effect of Three Factors on Gel Strength of Surimi Gels
2.1.2. Effect of Three Factors on Water-Holding Capacity of Surimi Gels
2.2. Orthogonal Test Result Analysis
2.3. Verification Test
2.4. Gel Strength and WHC
2.5. Textural Profile Analysis
2.6. Intermolecular Forces
2.7. Composition of Proteins
2.8. Microstructure
2.9. Sensory Evaluation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Single-Factor Test
Preparation of Surimi Gels with Different PHP Additions, Temperature, and Time
4.3. Determination of Gel Strength
4.4. Water-Holding Capacity (WHC)
4.5. Orthogonal Test
4.6. Validation and Comparison of Optimized Parameters
4.6.1. Preparation of Surimi Gel
4.6.2. Textural Profile Analysis
4.6.3. Chemical Interactions
Index of hydrogen bonds = SC − SB
Index of hydrophobic interactions = SD − SC
Index of disulfide bonds = SE − SD
4.6.4. Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.6.5. Scanning Electron Microscopy (SEM)
4.6.6. Sensory Evaluation
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, F.; Lin, L.; He, T.; Zhou, X.; Jiang, S.; Lu, J. Effect of Transglutaminase on Gel Properties of Surimi and Precocious Chinese Mitten Crab (Eriocheir sinensis) Meat. Food Hydrocoll. 2020, 98, 105261. [Google Scholar] [CrossRef]
- Ye, T.; Dai, H.; Lin, L.; Lu, J. Employment of Κ-carrageenan and High-Pressure Processing for Quality Improvement of Reduced NaCl Surimi Gels. J. Food Process Preserv. 2019, 43, e14074. [Google Scholar] [CrossRef]
- Yearbook. China Fisheries Statistical, China Agriculture Press: Beijing, China, 2022.
- Yi, S.; Ji, Y.; Guo, Z.; Zhu, J.; Xu, Y.; Li, X.; Li, J. Gel Properties and Flavor Characteristics of Blended Anchovy (Engraulis japonicus) Mince and Silver Carp (Hypophthalmichthys molitrix) Surimi. RSC Adv. 2020, 10, 6563–6570. [Google Scholar] [CrossRef]
- Jia, R.; Katano, T.; Yoshimoto, Y.; Gao, Y.; Nakazawa, N.; Osako, K.; Okazaki, E. Effect of Small Granules in Potato Starch and Wheat Starch on Quality Changes of Direct Heated Surimi Gels after Freezing. Food Hydrocoll. 2020, 104, 105732. [Google Scholar] [CrossRef]
- Yingchutrakul, M.; Wasinnitiwong, N.; Benjakul, S.; Singh, A.; Zheng, Y.; Mubango, E.; Luo, Y.; Tan, Y.; Hong, H. Asian Carp, an Alternative Material for Surimi Production: Progress and Future. Foods 2022, 11, 1318. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiong, S.; You, J.; Hu, Y.; Huang, Q.; Yin, T. Effects of Vacuum Chopping on Physicochemical and Gelation Properties of Myofibrillar Proteins from Silver Carp (Hypophthalmichthys molitrix). Food Chem. 2018, 245, 557–563. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012; ISBN 92-4-150483-8. [Google Scholar]
- Monto, A.R.; Li, M.; Wang, X.; Wijaya, G.Y.A.; Shi, T.; Xiong, Z.; Yuan, L.; Jin, W.; Li, J.; Gao, R. Recent Developments in Maintaining Gel Properties of Surimi Products under Reduced Salt Conditions and Use of Additives. Crit. Rev. Food Sci. Nutr. 2021, 62, 8518–8533. [Google Scholar] [CrossRef]
- Mi, H.; Li, Y.; Wang, C.; Yi, S.; Li, X.; Li, J. The Interaction of Starch-Gums and Their Effect on Gel Properties and Protein Conformation of Silver Carp Surimi. Food Hydrocoll. 2021, 112, 106290. [Google Scholar] [CrossRef]
- Cando, D.; Herranz, B.; Borderías, A.J.; Moreno, H.M. Different Additives to Enhance the Gelation of Surimi Gel with Reduced Sodium Content. Food Chem. 2016, 196, 791–799. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, Y.; Ye, T.; Nie, Y.; Jiang, S.; Lin, L.; Lu, J. Physicochemical Properties and Microstructure of Composite Surimi Gels: The Effects of Ultrasonic Treatment and Olive Oil Concentration. Ultrason. Sonochem 2022, 88, 106065. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, A.; Yang, R.; Jia, R.; Zhang, J.; Xu, D.; Yang, W. Chemical Interactions and Rheological Properties of Hairtail (Trichiurus haumela) Surimi: Effects of Chopping and Pressure. Food Biosci. 2020, 38, 100781. [Google Scholar] [CrossRef]
- Khaliq, R.; Tita, O.; Antofie, M.M.; Sava, C. Industrial Application of Psyllium: An Overview. Acta Univ. Cibiniensis 2015, 67, 210–214. [Google Scholar] [CrossRef]
- Deng, Z.; Meng, C.; Huang, H.; Song, S.; Fu, L.; Fu, Z. The Different Effects of Psyllium Husk and Orlistat on Weight Control, the Amelioration of Hypercholesterolemia and Non-Alcohol Fatty Liver Disease in Obese Mice Induced by a High-Fat Diet. Food Funct. 2022, 13, 8829–8849. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Kumar, P.; Raj, V. Psyllium Mucilage and Its Use in Pharmaceutical Field: An Overview. Curr. Synth. Syst. Biol. 2017, 5, 1. [Google Scholar] [CrossRef]
- Zhou, Y.; Dai, H.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Effect and Mechanism of Psyllium Husk (Plantago ovata) on Myofibrillar Protein Gelation. LWT 2021, 138, 110651. [Google Scholar] [CrossRef]
- Agrawal, R. Psyllium: A Source of Dietary Fiber. In Dietary Fibers; Waisundara, V.Y., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-83969-635-0. [Google Scholar]
- Garg, P.; Raghav, P.K.; Sharma, R.K.; Jasuja, N.D.; Sharma, R.; Agarwal, N. Development of Mucilaginous Spongy Dessert—A Herbal Rassogolla Prepared from Cow Milk. Int. J. Sci. Res. Publ. 2014, 41. [Google Scholar]
- Ladjevardi, Z.S.; Gharibzahedi, S.M.T.; Mousavi, M. Development of a Stable Low-Fat Yogurt Gel Using Functionality of Psyllium (Plantago ovata Forsk) Husk Gum. Carbohydr. Polym. 2015, 125, 272–280. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, L.; Yu, Y.; Zhu, H.; Wang, H.; Zhang, Y. Effect of Psyllium Husk Powder Addition on Quality of Meat Patties. J. Food Sci. Technol. 2019, 37, 42–49. [Google Scholar]
- Zhu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. Effects of Psyllium Husk Powder on the Gel Properties of Silver Carp (Hypophthalmichthys Molitrix) Surimi. J. Food Process Preserv. 2022, 46, e16452. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T.; Nuthong, P. Effect of Psyllium (Plantago ovata Forks) Husk on Characteristics, Rheological and Textural Properties of Threadfin Bream Surimi Gel. Foods 2021, 10, 1181. [Google Scholar] [CrossRef]
- Yi, S.; Li, Q.; Qiao, C.; Zhang, C.; Wang, W.; Xu, Y.; Mi, H.; Li, X.; Li, J. Myofibrillar Protein Conformation Enhance Gel Properties of Mixed Surimi Gels with Nemipterus virgatus and Hypophthalmichthys molitrix. Food Hydrocoll. 2020, 106, 105924. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, X.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Interactions and Phase Behaviors in Mixed Solutions of κ-Carrageenan and Myofibrillar Protein Extracted from Alaska Pollock Surimi. Food Res. Int. 2018, 105, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, X.; Ji, L.; Li, Z.; Wang, Y.; Xue, Y.; Xue, C. Phase Behaviors Involved in Surimi Gel System: Effects of Phase Separation on Gelation of Myofibrillar Protein and Kappa-Carrageenan. Food Res. Int. 2017, 100, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wu, D.; Wang, L.; Song, G.; Chi, R.; Ma, J.; Li, Z.; Wang, L.; Sun, W. Insoluble Dietary Fibers from Lentinus Edodes Stipes Improve the Gel Properties of Pork Myofibrillar Protein: A Water Distribution, Microstructure and Intermolecular Interactions Study. Food Chem. 2023, 411, 135386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, Y.; Xu, J.; Li, Z.; Xue, C. Effects of High-Temperature Treatment (⩾100 °C) on Alaska Pollock (Theragra chalcogramma) Surimi Gels. J. Food Eng. 2013, 115, 115–120. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, J.W. Negative Roles of Salt in Gelation Properties of Fish Protein Isolate. J. Food Sci. 2008, 73, C585–C588. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Mendes, R. Restructured Gel Products from Farmed Meagre (Argyrosomus Regius) Muscle: Effect of Low Salt Levels, Psyllium Fiber, and Chilled Storage. J. Aquat. Food Product. Technol. 2015, 24, 490–501. [Google Scholar] [CrossRef]
- Fu, X.; Hayat, K.; Li, Z.; Lin, Q.; Xu, S.; Wang, S. Effect of Microwave Heating on the Low-Salt Gel from Silver Carp (Hypophthalmichthys molitrix) Surimi. Food Hydrocoll. 2012, 27, 301–308. [Google Scholar] [CrossRef]
- Gao, X.; Xie, Y.; Yin, T.; Hu, Y.; You, J.; Xiong, S.; Liu, R. Effect of High Intensity Ultrasound on Gelation Properties of Silver Carp Surimi with Different Salt Contents. Ultrason. Sonochem 2021, 70, 105326. [Google Scholar] [CrossRef]
- Gao, X.; You, J.; Yin, T.; Xiong, S.; Liu, R. Simultaneous Effect of High Intensity Ultrasound Power, Time, and Salt Contents on Gelling Properties of Silver Carp Surimi. Food Chem. 2023, 403, 134478. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Bakry, A.M.; Xiong, S.; Yin, T.; Zhang, B.; Huang, J.; Liu, Z.; Huang, Q. Effect of Yeast β-Glucan on Gel Properties, Spatial Structure and Sensory Characteristics of Silver Carp Surimi. Food Hydrocoll. 2019, 88, 256–264. [Google Scholar] [CrossRef]
- You, S.; Yang, S.; Li, L.; Zheng, B.; Zhang, Y.; Zeng, H. Processing Technology and Quality Change during Storage of Fish Sausages with Textured Soy Protein. Foods 2022, 11, 3546. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, W. Effect of Setting Temperature on Glucono-δ-Lactone-Induced Gelation of Silver Carp Surimi. J. Sci. Food Agric. 2015, 95, 1528–1534. [Google Scholar] [CrossRef]
- Farahnaky, A.; Askari, H.; Majzoobi, M.; Mesbahi, G. The Impact of Concentration, Temperature and PH on Dynamic Rheology of Psyllium Gels. J. Food Eng. 2010, 100, 294–301. [Google Scholar] [CrossRef]
- He, X.; Lv, Y.; Li, X.; Yi, S.; Zhao, H.; Xu, Y.; Li, J. Effect of Oat β-glucan on Gel Properties and Protein Conformation of Silver Carp Surimi. J. Sci. Food Agric. 2023, 103, 3367–3375. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Q.; Shi, W. Ultrasound-Assisted Processing: Changes in Gel Properties, Water-Holding Capacity, and Protein Aggregation of Low-Salt Hypophthalmichthys molitrix Surimi by Soy Protein Isolate. Ultrason. Sonochem. 2023, 92, 106258. [Google Scholar] [CrossRef]
- Wang, Y.; Tu, X.; Shi, L.; Yang, H. Quality Characteristics of Silver Carp Surimi Gels as Affected by Okara. Int. J. Food Prop. 2023, 26, 49–64. [Google Scholar] [CrossRef]
- Buda, U.; Priyadarshini, M.B.; Majumdar, R.K.; Mahanand, S.S.; Patel, A.B.; Mehta, N.K. Quality Characteristics of Fortified Silver Carp Surimi with Soluble Dietary Fiber: Effect of Apple Pectin and Konjac Glucomannan. Int. J. Biol. Macromol. 2021, 175, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Y.; Dang, Y.; Cao, J.; Pan, D.; Guo, Y.; He, J. Water-Insoluble Dietary Fibers from Oats Enhance Gel Properties of Duck Myofibrillar Proteins. Food Chem. 2021, 344, 128690. [Google Scholar] [CrossRef]
- Cao, H.; Fan, D.; Jiao, X.; Huang, J.; Zhao, J.; Yan, B.; Zhou, W.; Zhang, W.; Zhang, H. Effects of Microwave Combined with Conduction Heating on Surimi Quality and Morphology. J. Food Eng. 2018, 228, 1–11. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Q.; Wang, P.; Song, C.; Ma, H.; Hong, P.; Zhou, C. Tapioca Starch Improves the Quality of Virgatus nemipterus Surimi Gel by Enhancing Molecular Interaction in the Gel System. Foods 2024, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Khalesi, H.; Zhao, Y.; Sun, C.; Lu, W.; Cao, Y.; Zhang, Y.; Kadkhodaee, R.; Fang, Y. Influence of Amyloid Fibril Length and Ionic Strength on WPI-Based Fiber-Hydrogel Composites: Microstructural, Rheological and Water Holding Properties. Food Hydrocoll. 2024, 148, 109499. [Google Scholar] [CrossRef]
- Shahsavani Mojarrad, L.; Rafe, A. Rheological Characteristics of Binary Composite Gels of Wheat Flour and High Amylose Corn Starch. J. Texture. Stud. 2018, 49, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Nishinari, K.; Fang, Y. Perception and Measurement of Food Texture: Solid Foods. J. Texture. Stud. 2018, 49, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Deng, T.; Wang, C.; Mi, H.; Yi, S.; Li, X.; Li, J. Effect of Hydrocolloids on Gel Properties and Protein Secondary Structure of Silver Carp Surimi. J. Sci. Food Agric. 2020, 100, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhao, X.; Li, X.; Huang, Q. Effects of Different Recovered Sarcoplasmic Proteins on the Gel Performance, Water Distribution and Network Structure of Silver Carp Surimi. Food Hydrocoll. 2022, 131, 107835. [Google Scholar] [CrossRef]
- Lee, C.H.; Chin, K.B. Evaluation of Various Salt Contents on Quality Characteristics with or without Curdlan of Pork Myofibrillar Protein Gels and the Development of Low-salt Pork Sausages. Int. J. Food Sci. Technol. 2019, 54, 550–557. [Google Scholar] [CrossRef]
- Liu, H.; Gao, L.; Ren, Y.; Zhao, Q. Chemical Interactions and Protein Conformation Changes during Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Formation. Int. J. Food Prop. 2014, 17, 1702–1713. [Google Scholar] [CrossRef]
- Zhong, Q.; Wang, Y.; Tian, Y.; Zhuang, Y.; Yang, H. Effects of Anthocyanins and Microbial Transglutaminase on the Physicochemical Properties of Silver Carp Surimi Gel. J. Texture. Stud. 2023, 54, 541–549. [Google Scholar] [CrossRef]
- Yu, N.; Xu, Y.; Jiang, Q.; Xia, W. Molecular Forces Involved in Heat-Induced Freshwater Surimi Gel: Effects of Various Bond Disrupting Agents on the Gel Properties and Protein Conformation Changes. Food Hydrocoll. 2017, 69, 193–201. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, Z.; Ding, Y. Changes in Physicochemical Properties and Protein Structure of Surimi Enhanced with Camellia Tea Oil. LWT 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Yin, T.; Yao, R.; Ullah, I.; Xiong, S.; Huang, Q.; You, J.; Hu, Y.; Shi, L. Effects of Nanosized Okara Dietary Fiber on Gelation Properties of Silver Carp Surimi. LWT 2019, 111, 111–116. [Google Scholar] [CrossRef]
- Buamard, N.; Benjakul, S. Cross-Linking Activity of Ethanolic Coconut Husk Extract Toward Sardine (Sardinella albella) Muscle Proteins. J. Food Biochem. 2017, 41, e12283. [Google Scholar] [CrossRef]
- Benjakul, S. Effect of Medium Temperature Setting on Gelling Characteristics of Surimi from Some Tropical Fish. Food Chem. 2003, 82, 567–574. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. The Effect of Salt on the Gelling Properties and Protein Phosphorylation of Surimi-Crabmeat Mixed Gels. Gels 2021, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.K.; Saha, A.; Dhar, B.; Maurya, P.K.; Roy, D.; Shitole, S.; Balange, A.K. Effect of Garlic Extract on Physical, Oxidative and Microbial Changes during Refrigerated Storage of Restructured Product from Thai Pangas (Pangasianodon hypophthalmus) Surimi. J. Food Sci. Technol. 2015, 52, 7994–8003. [Google Scholar] [CrossRef]
- Ma, X.-S.; Yi, S.-M.; Yu, Y.-M.; Li, J.-R.; Chen, J.-R. Changes in Gel Properties and Water Properties of Nemipterus virgatus Surimi Gel Induced by High-Pressure Processing. LWT Food Sci. Technol. 2015, 61, 377–384. [Google Scholar] [CrossRef]
- Hong, J.; Wu, J.; Chen, Y.; Jiang, Z.; Zhu, Y.; Li, Z.; Chen, X.; Ni, H.; Zheng, M. Effect of Black Tea Powder on Antioxidant Activity and Gel Characteristics of Silver Carp Fish Balls. Gels 2023, 9, 215. [Google Scholar] [CrossRef] [PubMed]
- Shahsavani Mojarrad, L.; Rafe, A.; Sadeghian, A.; Niazmand, R. Effects of High Amylose Corn Starch and Microbial Transglutaminase on the Textural and Microstructural Properties of Wheat Flour Composite Gels at High Temperatures. J. Texture. Stud. 2017, 48, 624–632. [Google Scholar] [CrossRef]
- Liang, F.; Zhu, Y.; Ye, T.; Jiang, S.; Lin, L.; Lu, J. Effect of Ultrasound Assisted Treatment and Microwave Combined with Water Bath Heating on Gel Properties of Surimi-Crabmeat Mixed Gels. LWT 2020, 133, 110098. [Google Scholar] [CrossRef]






| Serial No. | Temp. °C (A) | Time H (B) | Conc. % (C) | Gel Strength (g·cm) (yi1) | WHC (%) (yi2) | Gel Strength Degree (Yi1) | WHC Degree (Yi2) | Comb. Score (Yi) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 (35) | 1 (2) | 1 (0.1) | 213.20 | 57.04 | 0.00 | 0.00 | 0.00 |
| 2 | 1 | 2 (2.5) | 2 (0.2) | 273.23 | 68.68 | 0.59 | 0.70 | 6.45 |
| 3 | 1 | 3 (3) | 3 (0.3) | 311.65 | 73.64 | 0.97 | 1.00 | 9.85 |
| 4 | 2 (40) | 1 | 2 | 304.88 | 67.56 | 0.91 | 0.63 | 7.70 |
| 5 | 2 | 2 | 3 | 264.20 | 69.79 | 0.50 | 0.76 | 6.30 |
| 6 | 2 | 3 | 1 | 313.66 | 68.81 | 1.00 | 0.70 | 8.50 |
| 7 | 3 (45) | 1 | 3 | 226.76 | 64.50 | 0.13 | 0.40 | 2.65 |
| 8 | 3 | 2 | 1 | 237.39 | 65.54 | 0.20 | 0.50 | 3.50 |
| 9 | 3 | 3 | 2 | 281.70 | 62.16 | 0.68 | 0.30 | 4.90 |
| K1 | 16.30 | 10.20 | 12.00 | |||||
| K2 | 22.5 | 16.25 | 19.05 | |||||
| K3 | 11.05 | 23.25 | 18.80 | |||||
| Range | 11.45 | 13.05 | 7.05 | |||||
| Factors, major to minor | BAC | |||||||
| Optimal conditions | A2B3C2 |
| Serial No. | Temp °C (A) | Time H (B) | Conc. % (C) | Gel Strength (g·cm) (yi1) | WHC (%) (yi2) | Gel Strength Degree (Yi1) | WHC Degree (Yi2) | Comb. Score (Yi) |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 (35) | 1 (2) | 1 (0.1) | 415.25 | 77.81 | 0.82 | 0.81 | 8.15 |
| 2 | 1 | 2 (2.5) | 2 (0.2) | 449.05 | 80.92 | 1.00 | 1.00 | 10.0 |
| 3 | 1 | 3 (3) | 3 (0.3) | 436.79 | 80.85 | 0.93 | 0.99 | 9.60 |
| 4 | 2 (40) | 1 | 2 | 419 | 80.53 | 0.84 | 0.97 | 9.05 |
| 5 | 2 | 2 | 3 | 428.67 | 78.78 | 0.89 | 0.87 | 8.80 |
| 6 | 2 | 3 | 1 | 361.04 | 76.58 | 0.55 | 0.73 | 6.40 |
| 7 | 3 (45) | 1 | 3 | 273 | 78.18 | 0.10 | 0.83 | 4.65 |
| 8 | 3 | 2 | 1 | 252.56 | 64.35 | 0.00 | 0.00 | 0.00 |
| 9 | 3 | 3 | 2 | 260.49 | 69.66 | 0.04 | 0.32 | 1.80 |
| K1 | 27.75 | 21.85 | 14.55 | |||||
| K2 | 24.25 | 18.18 | 20.85 | |||||
| K3 | 6.45 | 17.8 | 23.05 | |||||
| Range | 21.3 | 4.05 | 8.5 | |||||
| Factors, major to minor | ACB | |||||||
| Optimal conditions | A1B1C3 |
| Salt % | Serial No. | Temp °C (A) | Time H (B) | Conc. % (C) | Gel Strength (g·cm) (yi1) | WHC (%) (yi2) | Gel Strength Degree (Yi1) | WHC Degree (Yi2) | Comb. Score (Yi) |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 10 | 2 (40) | 3 (3) | 2 (0.2) | 205.43 | 59.38 | 0.00 | 0.00 | 0.00 |
| 11 | 1 (35) | 3 | 3 (0.3) | 282.80 | 61.48 | 1.00 | 1.00 | 10.0 | |
| 1.0 | 12 | 1 | 1 (2) | 3 | 395.17 | 73.57 | 0.00 | 0.00 | 0.00 |
| 13 | 1 | 2 (2.5) | 2 | 433.5 | 75.83 | 1.00 | 1.00 | 10.0 |
| Sample | Hardness (g) | Adhesiveness (g. sec) | Springiness (%) | Cohesiveness (%) | Gumminess (g) | Chewiness (g) | Resilience (%) |
|---|---|---|---|---|---|---|---|
| OS1 | 8723.86 ± 76.72 b | −187.70 ± 101.42 a | 0.87 ± 0.00 bc | 0.68 ± 0.02 c | 5913.16 ± 212.90 c | 5133.96 ± 210.13 c | 0.36 ± 0.01 c |
| OSC1 | 8567.35 ± 97.71 bc | −159.67 ± 91.47 a | 0.86 ± 0.01 c | 0.70 ± 0.01 bc | 5988.61 ± 182.32 c | 5099.54 ± 121.23 c | 0.37 ± 0.01 c |
| TS1 | 8114.14 ± 208.24 d | −85.56 ± 71.65 a | 0.87 ± 0.01 b | 0.71 ± 0.01 b | 5763.86 ± 140.65 c | 5033.50 ± 134.20 c | 0.37 ± 0.37 c |
| OS2 | 9784.92 ± 151.78 a | −130.50 ± 109.94 a | 0.91 ± 0.02 a | 0.77 ± 0.03 a | 7564.82 ± 251.99 a | 6879.20 ± 172.43 a | 0.47 ± 0.00 a |
| OSC2 | 9606.17 ± 110.64 a | −149.13 ± 79.94 a | 0.91 ± 0.01 a | 0.79± 0.01 a | 7574.97 ± 181.92 a | 6906.84 ± 212.04 a | 0.48 ± 0.00 a |
| TS2 | 8310.89 ± 327.94 cd | −109.43 ± 104.51 a | 0.90 ± 0.02 a | 0.77 ± 0.01 a | 6414.06 ± 220.27 b | 5756.66 ± 183.40 b | 0.45 ± 0.00 b |
| Samples | Appearance | Aroma | Flavor | Texture | Overall Score |
|---|---|---|---|---|---|
| OS1 | 5.30 ± 0.86 ab | 4.95 ± 0.82 a | 5.10 ± 0.96 ab | 5.05 ± 0.82 bc | 5.41 ± 0.74 ab |
| OSC1 | 4.95 ± 1.05 b | 4.95 ± 0.68 a | 4.95 ± 0.94 b | 4.85 ± 1.03 c | 4.84 ± 1.30 b |
| TS1 | 4.95 ± 0.99 b | 4.80 ± 1.28 a | 5.05 ± 0.99 ab | 5.20 ± 1.00 bc | 5.46 ± 0.74 ab |
| OS2 | 5.60 ± 0.82 a | 5.20 ± 1.32 a | 5.70 ± 0.86 a | 6.00 ± 0.64 a | 5.70 ± 0.92 a |
| OSC2 | 5.05 ± 0.82 ab | 5.50 ± 0.76 a | 5.20 ± 1.15 ab | 5.55 ± 0.75 ab | 5.45 ± 0.60 ab |
| TS2 | 5.50 ± 0.60 ab | 5.15 ± 0.93 a | 5.40 ± 0.68 ab | 5.45 ± 0.99 abc | 5.65 ± 1.22 a |
| Level | Factors | ||
|---|---|---|---|
| Gelation Temperature (°C) | Gelation Time (h) | The Addition of PHP (%) | |
| 1 | 35.0 | 2.0 | 0.1 |
| 2 | 40.0 | 2.5 | 0.2 |
| 3 | 45.0 | 3.0 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.S.; Xia, L.; Li, Q.; Lu, Y.; Liu, S.; Lin, L.; Lu, J. Enhancing the Quality of Low-Salt Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Using Psyllium Husk Powder: An Orthogonal Experimental Approach. Gels 2024, 10, 247. https://doi.org/10.3390/gels10040247
Abbas MS, Xia L, Li Q, Lu Y, Liu S, Lin L, Lu J. Enhancing the Quality of Low-Salt Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Using Psyllium Husk Powder: An Orthogonal Experimental Approach. Gels. 2024; 10(4):247. https://doi.org/10.3390/gels10040247
Chicago/Turabian StyleAbbas, Muhammad Safeer, Lizhi Xia, Qiang Li, Yufeng Lu, Songkun Liu, Lin Lin, and Jianfeng Lu. 2024. "Enhancing the Quality of Low-Salt Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Using Psyllium Husk Powder: An Orthogonal Experimental Approach" Gels 10, no. 4: 247. https://doi.org/10.3390/gels10040247
APA StyleAbbas, M. S., Xia, L., Li, Q., Lu, Y., Liu, S., Lin, L., & Lu, J. (2024). Enhancing the Quality of Low-Salt Silver Carp (Hypophthalmichthys molitrix) Surimi Gel Using Psyllium Husk Powder: An Orthogonal Experimental Approach. Gels, 10(4), 247. https://doi.org/10.3390/gels10040247