Single-Handed Helical Polybissilsesquioxane Nanotubes and Mesoporous Nanofibers Prepared by an External Templating Approach Using Low-Molecular-Weight Gelators
Abstract
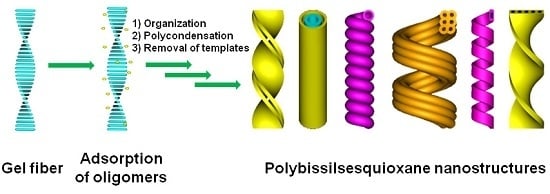
:1. Introduction
2. Preparation and Formation Mechanism
2.1. Single-Handed Helical Mesoporous Nanofibers
2.2. Single-Handed Helical Nanotubes
3. Optical Activity of the Aromatic Ring-Bridged Polybissilsesquioxanes
4. TEM, XRD, and N2 Sorption Characterizations
5. Other Nanotubes Prepared Using LMWGs
6. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, H.; Luo, Z.; Wang, Y.; He, T.; Yang, C.; Ren, C.; Ma, L.; Gong, C.; Li, X.; Yang, Z. Enzyme-catalyzed formation of supramolecular hydrogels as promising vaccine adjuvants. Adv. Funct. Mater. 2016, 26, 1822–1829. [Google Scholar] [CrossRef]
- Ren, C.; Wang, H.; Mao, D.; Zhang, X.; Fengzhao, Q.; Shi, Y.; Ding, D.; Kong, D.; Wang, L.; Yang, Z. When molecular probes meet self-assembly: An enhanced quenching effect. Angew. Chem. Int. Ed. 2015, 54, 4823–4827. [Google Scholar] [CrossRef] [PubMed]
- Pont, G.; Chen, L.; Spiller, D.G.; Adams, D.J. The effect of polymer additives on the rheological properties of dipeptide hydrogelators. Soft Matter 2012, 8, 7797–7802. [Google Scholar] [CrossRef]
- Abul-Haija, Y.M.; Ulijn, R.V. Sequence peptide-polysaccharide nanostructures by biocatalytic self-assembly. Biomacromolecules 2015, 16, 3473–3479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazos, E.; Sleep, E.; Pérez, C.M.R.; Lee, S.S.; Tantakitti, F.; Stupp, S.I. Nucleation and growth of ordered arrays of silver nanoparticles on peptide nanofibers: Hybrid nanostructures with antimicrobial properties. J. Am. Chem. Soc. 2016, 138, 5507–5510. [Google Scholar] [CrossRef] [PubMed]
- Guterman, T.; Kornreich, M.; Stern, A.; Adler-Abramovich, L.; Porath, D.; Beck, R.; Shimon, L.J.W.; Gazit, E. Formation of bacterial pilus-like nanofibers by designed minimalistic self-assembling peptides. Nat. Commun. 2016, 7, 13482. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.O.; Michaels, T.C.T.; Levin, A.; Gazit, E.; Dobson, C.M.; Buell, A.K.; Knowles, T.P.J. Synthesis of nonoequilibrium supramolecular peptide polymers on a microfluidic platform. J. Am. Chem. Soc. 2016, 138, 9589–9596. [Google Scholar] [CrossRef] [PubMed]
- Pashuck, E.T.; Stupp, S.I. Direct observation of morphological transformation from twisted ribbons into helical ribbons. J. Am. Chem. Soc. 2010, 132, 8819–8821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Fu, Y.; Lin, S.; Yang, Y. Solvent-induced handedness inversion of dipeptide sodium salts derived from alanine. Langmuir 2013, 29, 9721–9726. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, Y.; Li, B.; Yang, Y. Control of the handedness of self-assemblies of dipeptides by the chirality of phenylalanine and steric hindrance of phenylglycine. Langmuir 2016, 32, 7420–7426. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; John, G.; Yoshida, K.; Shimizu, T. Self-assembling structures of long-chain phenyl glucoside influenced by the introduction of double bonds. J. Am. Chem. Soc. 2002, 124, 10674–10675. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Do, Y.; Lee, Y.A.; Shimizu, T. Self-assembling structures of long-chain sugar-based amphiphiles influenced by the introduction of double bonds. Chem. Eur. J. 2005, 11, 5538–5544. [Google Scholar] [CrossRef] [PubMed]
- Dawn, A.; Fujita, N.; Haraguchi, S.; Sada, K.; Shinkai, S. An organogel system can control the stereochemical course of anthracene photodimerization. Chem. Commun. 2009, 45, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhang, L.; Cao, H.; Wang, T.; Zhu, X.; Jiang, J.; Liu, M. Self-assembly of copper(II) ion-mediated nanotube and its supramolecular chiral catalytic behavior. Langmuir 2011, 27, 13847–13853. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Llansola, F.; Miravet, J.F.; Escuder, B. Single amino acid based thixotropic hydrogel formation and pH-dependent morphological change of gel nanofibers. Chem. Eur. J. 2010, 16, 8480–8486. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Nakashima, K.; Sano, M.; Kanekiyo, Y.; Inoue, K.; Hojo, J.; Shinkai, S. Organic gels are useful as a template for the preparation of hollow fiber silica. Chem. Commun. 1998, 34, 1477–1479. [Google Scholar] [CrossRef]
- Jung, J.H.; Ono, Y.; Hanabusa, K.; Shinkai, S. Creation of both right-handed and left-handed silica structures by sol−gel transcription of organogel fibers comprised of chiral diaminocyclohexane derivatives. J. Am. Chem. Soc. 2000, 122, 5008–5009. [Google Scholar] [CrossRef]
- Bommel, K.J.C.; Friggeri, A.; Shinkai, S. Organic templates for the generation of inorganic materials. Angew. Chem. Int. Ed. 2003, 42, 980–999. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Hamasaki, N.; Suzuki, M.; Kimura, M.; Shirai, H.; Hanabusa, K. Preparation of helical transition-metal oxide tubes using organogelators as structure-directing agents. J. Am. Chem. Soc. 2002, 124, 6550–6551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Huo, H.; Huang, Z.; Li, Y.; Li, B.; Yang, Y. Preparation of helical mesoporous tantalum oxide nanotubes through a sol–gel transcription approach. Chem. Asian J. 2013, 8, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Huo, H.; Li, Y.; Li, B.; Yang, Y. Preparation of helical titania nanotubes using a sol–gel transcription approach. Mater. Lett. 2012, 88, 23–26. [Google Scholar] [CrossRef]
- Huo, H.; Wang, S.; Lin, S.; Li, Y.; Li, B.; Yang, Y. Chiral zirconia nanotubes prepared through a sol–gel transcription approach. J. Mater. Chem. A. 2014, 2, 333–338. [Google Scholar] [CrossRef]
- Zhang, C.; Huo, H.; Li, Y.; Li, B.; Yang, Y. Preparation of helical CdS nanotubes using a sol–gel transcription approach. Mater. Lett. 2013, 102–103, 50–52. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Tang, X.; Li, B.; Zhang, C.; Yang, Y. Preparation of single-handed helical carbonaceous nanotubes using 3-aminophenol-formaldehyde resin. RSC Adv. 2015, 5, 39946–39951. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Zhao, H.; Pei, X.; Bi, L.; Hanabusa, K.; Yang, Y. From branched self-assemblies to branched mesoporous silica nanoribbons. Chem. Commun. 2008, 44, 6366–6368. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Suzuki, M.; Owa, S.; Shirai, H.; Hanabusa, K. Control of mesoporous silica nanostructures and pore-architectures using a thickener and a gelator. J. Am. Chem. Soc. 2007, 129, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Suzuki, M.; Shirai, H.; Kurose, A.; Hanabusa, K. Nanofiberization of inner helical mesoporous silica using chiral gelator as template under a shear flow. Chem. Commun. 2005, 41, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Suzuki, M.; Owa, S.; Shirai, H.; Hanabusa, K. Preparation of helical nanostructures using chiral cationic surfactants. Chem. Commun. 2005, 41, 4462–4464. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Suzuki, M.; Owa, S.; Shirai, H.; Hanabusa, K. Control of helical silica nanostructures using a chiral surfactant. J. Mater. Chem. 2006, 16, 1644–1650. [Google Scholar] [CrossRef]
- Corriu, R.J.P. Ceramics and nanostructures from molecular precursors. Angew. Chem. Int. Ed. 2000, 39, 1376–1398. [Google Scholar] [CrossRef]
- Park, S.S.; Lee, C.H.; Cheon, J.H.; Choe, S.J.; Park, D.H. Morphological control of periodic mesoporous organosilica with agitation. Bull. Korean Chem. Soc. 2001, 22, 948–952. [Google Scholar]
- Yang, S.; Zhao, L.; Yu, C.; Zhou, X.; Tang, J.; Yuan, P.; Chen, D.; Zhao, D. On the origin of helical mesostructures. J. Am. Chem. Soc. 2006, 128, 10460–10466. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhao, L.; Liu, N.; Wei, G.; Zhang, Y.; Wang, Y.; Yu, C. Periodic mesoporous organosilicas with helical and concentric circular pore architectures. Chem. Eur. J. 2009, 15, 11319–11325. [Google Scholar] [CrossRef] [PubMed]
- Rambaud, F.; Vallé, K.; Thibaud, S.; Julián-López, B.; Sanchez, C. Hybrid nanofiber growth: One-pot synthesis of functional helicoidal hybrid organic–inorganic nanofibers with periodically organized mesoporosity. Adv. Funct. Mater. 2009, 19, 2896–2905. [Google Scholar] [CrossRef]
- Zhou, L.; Hong, G.; Qi, L.; Lu, Y. Seeding-growth of helical mesoporous silica nanofibers templated by achiral cationic surfactant. Langmuir 2009, 25, 6040–6044. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhao, L.; Ying, Y. Entropy-driven helical mesostructure formation with achiral cationic surfactant templates. Adv. Mater. 2007, 19, 2454–2459. [Google Scholar] [CrossRef]
- Li, Y.; Bi, L.; Wang, S.; Chen, Y.; Li, B.; Zhu, X.; Yang, Y. Preparation of helical mesoporous ethylene-silica nanofibers with lamellar mesopores on the surfaces. Chem. Commun. 2010, 46, 2680–2682. [Google Scholar] [CrossRef]
- Meng, X.; Yokoi, T.; Lu, D.; Tataumi, T. Synthesis and characterization of chiral periodic mesoporous organosilicas. Angew. Chem. Int. Ed. 2007, 46, 7796–7798. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yuan, P.; Zhao, L.; Zhou, L.; Wang, Y.; Yu, C. Synthesis of enantiomorphic excessive helical mesoporous silicas using chiral molecular dopants. Chem. Lett. 2008, 37, 1160–1161. [Google Scholar] [CrossRef]
- Moreau, J.J.E.; Vellutini, L.; Wong Chi Man, M.; Bied, C. New hybrid organic-inorganic solids with helical morphology via H-bond mediated sol-gel hydrolysis of silyl derivatives of chiral (R,R)- or (S,S)-diureidocyclohexane. J. Am. Chem. Soc. 2001, 123, 1509–1510. [Google Scholar] [CrossRef]
- Xu, Q.; Moreau, J.J.E.; Wong Chi Man, M. Influence of alkylene chain length on the morphology of chiral bridged silsesquioxanes. J. Sol-Gel Sci. Technol. 2004, 32, 111–115. [Google Scholar]
- Yang, Y.; Nakazawa, M.; Suzuki, M.; Kimura, M.; Shirai, H.; Hanabusa, K. Formation of helical hybrid silica bundles. Chem. Mater. 2004, 16, 3791–3793. [Google Scholar] [CrossRef]
- Yang, Y.; Nakazawa, M.; Suzuki, M.; Shirai, H.; Hanabusa, K. Fabrication of helical hybrid silica bundles. J. Mater. Chem. 2007, 17, 2936–2943. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, S.; Qin, J.; Li, Y.; Li, B.; Yang, Y. Helical polybissilsesquioxane bundles prepared using a self-templating approach. Chirality 2016, 28, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Suzuki, M.; Fukui, H.; Shirai, H.; Hanabusa, K. Preparation of helical mesoporous silica and hybrid silica nanofibers using hydrogelator. Chem. Mater. 2006, 18, 1324–1329. [Google Scholar] [CrossRef]
- Wu, X.; Ji, S.; Li, Y.; Li, B.; Zhu, X.; Hanabusa, K.; Yang, Y. Helical transfer through nonlocal interactions. J. Am. Chem. Soc. 2009, 131, 5986–5993. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Y.; Li, B.; Yang, Y. Preparation of single-handed helical carbon/silica and carbonaceous nanotubes using 4,4′-biphenylene bridged polybissilsesquioxane. Chem. Asian J. 2013, 8, 2714–2720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, B.; Li, Y.; Wang, M.; Yang, Y. Optical activity of SiC nanoparticles prepared from single-handed helical 4,4′-biphenylene-bridged polybissilsesquioxane nanotubes. New J. Chem. 2015, 39, 8424–8429. [Google Scholar] [CrossRef]
- Li, B.; Xu, Z.; Zhuang, W.; Chen, Y.; Wang, S.; Li, Y.; Wang, M.; Yang, Y. Characterization of 4,4′-biphenylene-silicas and a chiral sensor for silicas. Chem. Commun. 2011, 47, 11495–11497. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhuang, W.; Li, B.; Wu, L.; Wang, S.; Li, Y.; Yang, Y. Helical periodic mesoporous 1,4-phenylene-silica nanorods with chiral crystalline walls. Chem. Commun. 2011, 47, 7215–7217. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Xiao, M.; Wang, M.; Huang, Z.; Li, B.; Yang, Y. Chirality of the 1,4-phenylene–silica nanoribbons at the nano and angstrom levels. Nanotechnology 2013, 24, 035603. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Wu, X.; Zhu, X.; Suzuki, M.; Hanabusa, K.; Yang, Y. Hybrid silica tubes with chiral walls. Chem. Commun. 2008, 44, 4948–4950. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, B.; Chen, Y.; Wu, X.; Zhang, J.; Li, Y.; Hanabusa, K.; Yang, Y. Transfer helix and chirality to 1,4-phenylene silica nanostructures. Mater. Chem. Phys. 2009, 118, 135–141. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Chen, Y.; Zhang, M.; Wang, S.; Li, Y.; Yang, Y. Preparation of chiral 4,4′-biphenylene-silica nanoribbons. J. Chin. Chem. 2009, 27, 1860–1862. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, L.; Zhou, F.; Chen, Y.; Li, B.; Yang, Y. Handedness inversion in preparing chiral 4,4'-biphenylene-silica nanostructures. Nanotechnology 2011, 22, 135605. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Wang, H.; Zhang, M.; Chen, Y.; Li, B.; Yang, Y. Nanofabrication of helical hybrid silica nanotubes using anionic gelators. Mater. Chem. Phys. 2010, 124, 609–613. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Li, Y.; Zhuang, W.; Zhu, Z.; Chen, Y.; Li, B.; Yang, Y. Formation of helical organic–inorganic hybrid silica nanotubes using a chiral anionic gelator. J. Nanosci. Nanotechnol. 2011, 11, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Fu, Y.; Sang, Y.; Li, Y.; Li, B.; Yang, Y. Characterization of chiral carbonaceous nanotubes prepared from four coiled tubular 4,4′-biphenylene-silica nanoribbons. AIMS Mater. Sci. 2014, 1, 1–10. [Google Scholar]
- Liu, D.; Li, B.; Guo, Y.; Li, Y.; Yang, Y. Inner surface chirality of single-handed twisted carbonaceous tubular nanoribbons. Chirality 2015, 27, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Guan, S.; Ohsuna, T.; Terasaki, O. An ordered mesoporous organosilica hybrid material with a crystal-like wall structure. Nature 2002, 416, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Kapoor, M.P.; Inagaki, S. Sulfuric acid-functionalized mesoporous benzene-silica with a molecular-scale periodicity in the walls. J. Am. Chem. Soc. 2002, 124, 9694–9695. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Li, Y.; Wang, S.; Xu, Z.; Chen, Y.; Li, B.; Zhu, X.; Zhu, G.; Yang, Y. Artificial frustule prepared through a single-templating approach. Chem. Commun. 2010, 46, 8410–8412. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhao, Y.; Wu, L.; Li, Y.; Li, B.; Wang, M.; Yang, Y. A chirality indicator for the surfaces of the silica nanotubes. J. Nanosci. Nanotechnol. 2013, 13, 5732–5735. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Zhao, L.; Liu, N.; Wei, G.; Wang, Y.; Auchterlonie, G.J.; Drennan, J.; Lu, G.Q.; Zhou, J.; Yu, C. Evolution of helical mesostructures. Chem. Eur. J. 2010, 16, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, C.; Li, B.; Li, Y.; Yang, Y. Preparation and characterization of single-handed twisted platinum tubular nanoribbons. Mater. Lett. 2014, 133, 147–150. [Google Scholar] [CrossRef]
- Wang, S.; Li, R.; Zhang, C.; Li, Y.; Li, B.; Yang, Y. Formation and asymmetric catalysis of C- and 8-shaped titania tubes. Mater. Lett. 2013, 106, 71–74. [Google Scholar] [CrossRef]








© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Yang, Y. Single-Handed Helical Polybissilsesquioxane Nanotubes and Mesoporous Nanofibers Prepared by an External Templating Approach Using Low-Molecular-Weight Gelators. Gels 2017, 3, 2. https://doi.org/10.3390/gels3010002
Hu J, Yang Y. Single-Handed Helical Polybissilsesquioxane Nanotubes and Mesoporous Nanofibers Prepared by an External Templating Approach Using Low-Molecular-Weight Gelators. Gels. 2017; 3(1):2. https://doi.org/10.3390/gels3010002
Chicago/Turabian StyleHu, Jing, and Yonggang Yang. 2017. "Single-Handed Helical Polybissilsesquioxane Nanotubes and Mesoporous Nanofibers Prepared by an External Templating Approach Using Low-Molecular-Weight Gelators" Gels 3, no. 1: 2. https://doi.org/10.3390/gels3010002
APA StyleHu, J., & Yang, Y. (2017). Single-Handed Helical Polybissilsesquioxane Nanotubes and Mesoporous Nanofibers Prepared by an External Templating Approach Using Low-Molecular-Weight Gelators. Gels, 3(1), 2. https://doi.org/10.3390/gels3010002