Crosslinker-Based Regulation of Swelling Behavior of Poly(N-isopropylacrylamide) Gels in a Post-Polymerization Crosslinking System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Prepolymer Synthesis
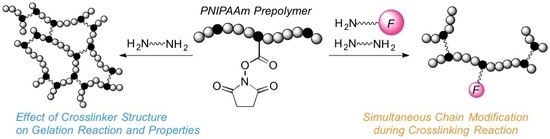
2.2. Effect of Crosslinker Structure on Gelation Behavior by PPC
2.3. Control of Crosslinking Density by SMC Method
2.4. Control of Response Temperature by SMC Method
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Prepolymer Synthesis
4.3. PPC-Gel Synthesis
4.4. SMC Reaction
4.5. Measurements
Author Contributions
Funding
Conflicts of Interest
References
- Osada, Y.; Gong, J. Stimuli-responsive polymer gels and their application to chemomechanical systems. Prog. Polym. Sci. 1993, 18, 187–226. [Google Scholar] [CrossRef]
- Osada, Y.; Gong, J.-P. Soft and Wet Materials: Polymer Gels. Adv. Mater. 1998, 10, 827–837. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interfaces Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Akiyoshi, K. Nanogel engineering for new nanobiomaterials: From chaperoning engineering to biomedical applications. Chem. Rec. 2010, 10, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Diaz Diaz, D.; Kuhbeck, D.; Koopmans, R.J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Hiratani, H.; Alvarez-Lorenzo, C.; Chuang, J.; Guney, O.; Grosberg, A.Y.; Tanaka, T. Effect of Reversible Cross-linker, N,N′ -Bis(acryloyl)cystamine, on Calcium Ion Adsorption by Imprinted Gels. Langmuir 2001, 17, 4431–4436. [Google Scholar] [CrossRef]
- Murthy, N.; Thng, Y.X.; Schuck, S.; Xu, M.C.; Fréchet, J.M.J. A Novel Strategy for Encapsulation and Release of Proteins: Hydrogels and Microgels with Acid-Labile Acetal Cross-Linkers. J. Am. Chem. Soc. 2002, 124, 12398–12399. [Google Scholar] [CrossRef]
- Burek, M.; Czuba, Z.P.; Waskiewicz, S. Novel acid-degradable and thermo-sensitive poly(N-isopropylacrylamide) hydrogels cross-linked by α,α-trehalose diacetals. Polymer 2014, 55, 6460–6470. [Google Scholar] [CrossRef]
- Otsuka, H. Reorganization of polymer structures based on dynamic covalent chemistry: Polymer reactions by dynamic covalent exchanges of alkoxyamine units. Polym. J. 2013, 45, 879–891. [Google Scholar] [CrossRef]
- Imato, K.; Otsuka, H. Reorganizable and stimuli-responsive polymers based on dynamic carbon–carbon linkages in diarylbibenzofuranones. Polymer 2018, 137, 395–413. [Google Scholar] [CrossRef]
- Patrickios, C.S.; Georgiou, T.K. Covalent Amphiphilic Polymer Networks. Curr. Opin. Colloid Interface Sci. 2003, 8, 76–85. [Google Scholar] [CrossRef]
- Erdodi, G.; Kennedy, J.P. Amphiphilic Conetworks: Definition, Synthesis, Applications. Prog. Polym. Sci. 2006, 31, 1–18. [Google Scholar] [CrossRef]
- Kali, G.; Iván, B. Poly(methacrylic acid)-l-Polyisobutylene Amphiphilic Conetworks by Using an Ethoxyethyl-Protected Comonomer: Synthesis, Protecting Group Removal in the Cross-Linked State, and Characterization. Macromol. Chem. Phys. 2015, 216, 605–613. [Google Scholar] [CrossRef]
- Fodor, C.; Kali, G.; Iván, B. Poly(N-vinylimidazole)-l-Poly(tetrahydrofuran) Amphiphilic Conetworks and Gels: Synthesis, Characterization, Thermal and Swelling Behavior. Macromolecules 2011, 44, 4496–4502. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Nutan, B.; Raval, I.H.; Jewrajka, S.K. Self-Assembly of Partially Alkylated Dextran-graft-poly[(2-dimethylamino)ethyl methacrylate] Copolymer Facilitating Hydrophobic/Hydrophilic Drug Delivery and Improving Conetwork Hydrogel Properties. Biomacromolecules 2018, 19, 1142–1153. [Google Scholar] [CrossRef]
- Guzman, G.; Bhaway, S.M.; Nugay, T.; Vogt, B.D.; Cakmak, M. Transport-Limited Adsorption of Plasma Proteins on Bimodal Amphiphilic Polymer Co-Networks: Real-Time Studies by Spectroscopic Ellipsometry. Langmuir 2017, 33, 2900–2910. [Google Scholar] [CrossRef]
- Tokuyama, H.; Ishihara, N.; Sakohara, S. Effects of synthesis-solvent on swelling and elastic properties of poly(N-isopropylacrylamide) hydrogels. Eur. Polym. J. 2007, 43, 4975–4982. [Google Scholar] [CrossRef]
- Abdurrahmanoglu, S.; Okay, O. Homogeneous Poly(acrylamide) Hydrogels Made by Large Size, Flexible Dimethacrylate Cross-Linkers. Macromolecules 2008, 41, 7759–7761. [Google Scholar] [CrossRef]
- Ida, S. Structural design of vinyl polymer hydrogels utilizing precision radical polymerization. Polym. J. 2019, 51, 803–812. [Google Scholar] [CrossRef]
- Ida, S.; Katsurada, A.; Yoshida, R.; Hirokawa, Y. Effect of reaction conditions on poly(N-isopropylacrylamide) gels synthesized by post-polymerization crosslinking system. React. Funct. Polym. 2017, 115, 73–80. [Google Scholar] [CrossRef]
- Ida, S.; Kawahara, T.; Kawabata, H.; Ishikawa, T.; Hirokawa, Y. Effect of Monomer Sequence along Network Chains on Thermoresponsive Properties of Polymer Gels. Gels 2018, 4, 22. [Google Scholar]
- Ida, S.; Kitanaka, H.; Ishikawa, T.; Kanaoka, S.; Hirokawa, Y. Swelling properties of thermoresponsive/hydrophilic co-networks with functional crosslinked domain structures. Polym. Chem. 2018, 9, 1701–1709. [Google Scholar] [CrossRef]
- Ida, S.; Morimura, M.; Kitanaka, H.; Hirokawa, Y.; Kanaoka, S. Swelling and mechanical properties of thermoresponsive/hydrophilic conetworks with crosslinked domain structures prepared from various triblock precursors. Polym. Chem. 2019, 10, 6122–6130. [Google Scholar] [CrossRef]
- Martinez, M.V.; Molina, M.; Barbero, C.A. Poly(N-isopropylacrylamide) Cross-Linked Gels as Intrinsic Amphiphilic Materials: Swelling Properties Used to Build Novel Interphases. J. Phys. Chem. B 2018, 122, 9038–9048. [Google Scholar] [CrossRef]
- Pollak, A.; Blumenfeld, H.; Wax, M.; Baughn, R.L.; Whitesides, G.M. Enzyme immobilization by condensation copolymerization into crosslinked polyacrylamide gels. J. Am. Chem. Soc. 1980, 102, 6324–6336. [Google Scholar] [CrossRef]







| Code | Feed Concentration [NIPAAm]:[NHSA] (mM) | Conversion [%] | NHSA Content [%] 2 | Mn3 | Mw/Mn3 | |
|---|---|---|---|---|---|---|
| NIPAAm | NHSA | |||||
| PN2 | 1980:20 | 37 | 58 | 1.8 | 91,000 | 1.51 |
| PN6 | 1970:30 | 52 | 91 | 5.8 | 85,000 | 1.58 |
| PN29 | 1500:500 | 73 | 96 | 29 | 51,000 | 1.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ida, S.; Katsurada, A.; Tsujio, M.; Nakamura, M.; Hirokawa, Y. Crosslinker-Based Regulation of Swelling Behavior of Poly(N-isopropylacrylamide) Gels in a Post-Polymerization Crosslinking System. Gels 2020, 6, 2. https://doi.org/10.3390/gels6010002
Ida S, Katsurada A, Tsujio M, Nakamura M, Hirokawa Y. Crosslinker-Based Regulation of Swelling Behavior of Poly(N-isopropylacrylamide) Gels in a Post-Polymerization Crosslinking System. Gels. 2020; 6(1):2. https://doi.org/10.3390/gels6010002
Chicago/Turabian StyleIda, Shohei, Akimitsu Katsurada, Mitsuhiro Tsujio, Motoharu Nakamura, and Yoshitsugu Hirokawa. 2020. "Crosslinker-Based Regulation of Swelling Behavior of Poly(N-isopropylacrylamide) Gels in a Post-Polymerization Crosslinking System" Gels 6, no. 1: 2. https://doi.org/10.3390/gels6010002
APA StyleIda, S., Katsurada, A., Tsujio, M., Nakamura, M., & Hirokawa, Y. (2020). Crosslinker-Based Regulation of Swelling Behavior of Poly(N-isopropylacrylamide) Gels in a Post-Polymerization Crosslinking System. Gels, 6(1), 2. https://doi.org/10.3390/gels6010002