Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels
Abstract
:1. Introduction
2. Results and Discussion
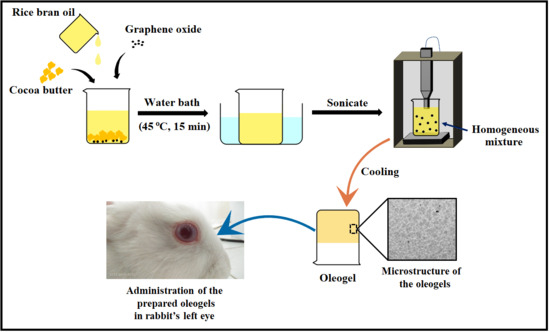
2.1. Preparation of the Oleogels
2.2. Bright-Field Microscopy Analysis
2.3. FTIR Analysis
2.4. XRD Analysis
2.5. Thermal Analysis
2.5.1. Crystallization Kinetics
2.5.2. DSC Analysis
2.6. Mechanical Analysis
2.7. Drug Diffusion and Permeation Studies
2.8. Antimicrobial Analysis
2.9. Ocular Irritation Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Oleogels
3.3. Characterization of the Oleogels
3.4. In Vitro Drug Diffusion Study
3.5. Biological Studies
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pehlivanoğlu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar]
- Davidovich-Pinhas, M. Oleogels. In Polymeric Gels; Elsevier: Amsterdam, The Netherlands, 2018; pp. 231–249. [Google Scholar]
- Yılmaz, E.; Öğütcü, M. The texture, sensory properties and stability of cookies prepared with wax oleogels. Food Funct. 2015, 6, 1194–1204. [Google Scholar] [PubMed]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.S.; Lee, S. Utilization of oleogels as a replacement for solid fat in aerated baked goods: Physicochemical, rheological, and tomographic characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [PubMed]
- Fayaz, G.; Goli, S.A.H.; Kadivar, M.; Valoppi, F.; Barba, L.; Calligaris, S.; Nicoli, M.C. Potential application of pomegranate seed oil oleogels based on monoglycerides, beeswax and propolis wax as partial substitutes of palm oil in functional chocolate spread. LWT 2017, 86, 523–529. [Google Scholar]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar]
- Munk, M.B.; Munk, D.M.; Gustavsson, F.; Risbo, J. Using Ethylcellulose to Structure Oil Droplets in Ice Cream Made with High Oleic Sunflower Oil. J. Food Sci. 2018, 83, 2520–2526. [Google Scholar] [PubMed]
- Hughes, N.; Rush, J.W.; Marangoni, A.G. Feeding study of 12-hydroxystearic acid oleogels. In Edible Oleogels, 2 ed.; Marangoni, A.G., Garti, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 381–399. [Google Scholar]
- Jiang, Y.; Liu, L.; Wang, B.; Sui, X.; Zhong, Y.; Zhang, L.; Mao, Z.; Xu, H. Cellulose-rich oleogels prepared with an emulsion-templated approach. Food Hydrocoll. 2018, 77, 460–464. [Google Scholar]
- Ash, D.; Majee, S.B.; Biswas, G.R. Oleogels of olive oil and soybean oil for topical drug delivery: A comparative analysis. Int. J. Pharm. Biol. Sci. 2019, 11, 4–10. [Google Scholar]
- Mohanty, B.; Pal, K.; Quereshi, D.; Nayak, S.K.; Rathnam, V.S.S.; Banerjee, I.; Anis, A.; Barik, C.S.; Sarkar, P.; Rout, S.K. Oleogels Based on Palmitic Acid and Safflower Oil: Novel Formulations for Ocular Drug Delivery of Voriconazole. Eur. J. Lipid Sci. Technol. 2020, 122, 1900288. [Google Scholar]
- Masotta, N.E.; Martinefski, M.R.; Lucangioli, S.; Rojas, A.M.; Tripodi, V.P. High-dose coenzyme Q10-loaded oleogels for oral therapeutic supplementation. Int. J. Pharm. 2019, 556, 9–20. [Google Scholar]
- Macoon, R.; Robey, M.; Chauhan, A. In Vitro release of hydrophobic drugs by oleogel rods with biocompatible gelators. Eur. J. Pharm. Sci. 2020, 152, 105413. [Google Scholar] [PubMed]
- Hamed, R.; AbuRezeq, A.A.; Tarawneh, O. Development of hydrogels, oleogels, and bigels as local drug delivery systems for periodontitis. Drug Dev. Ind. Pharm. 2018, 44, 1488–1497. [Google Scholar] [PubMed]
- Fréchou, M.; Zhang, S.; Liere, P.; Delespierre, B.; Soyed, N.; Pianos, A.; Schumacher, M.; Mattern, C.; Guennoun, R. Intranasal delivery of progesterone after transient ischemic stroke decreases mortality and provides neuroprotection. Neuropharmacology 2015, 97, 394–403. [Google Scholar] [PubMed]
- Wang, D.; Zhao, J.; Liu, X.; Sun, F.; Zhou, Y.; Teng, L.; Li, Y. Parenteral thermo-sensitive organogel for schizophrenia therapy, in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2014, 60, 40–48. [Google Scholar]
- Macoon, R.; Guerriero, T.; Chauhan, A. Extended release of dexamethasone from oleogel based rods. J. Colloid Interface Sci. 2019, 555, 331–341. [Google Scholar]
- Kongor, J.E.; Hinneh, M.; Van de Walle, D.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile—A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar]
- Oracz, J.; Zyzelewicz, D.; Nebesny, E. The content of polyphenolic compounds in cocoa beans (Theobroma cacao L.), depending on variety, growing region, and processing operations: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1176–1192. [Google Scholar]
- Buscato, M.H.M.; Grimaldi, R.; Kieckbusch, T.G. Cocoa butter symmetrical monounsaturated triacylglycerols: Separation by solvent fractionation and application as crystallization modifier. J. Food Sci. Technol. 2017, 54, 3260–3267. [Google Scholar]
- Biswas, N.; Cheow, Y.L.; Tan, C.P.; Siow, L.F. Physicochemical Properties of Enzymatically Produced Palm-Oil-Based Cocoa Butter Substitute (CBS) With Cocoa Butter Mixture. Eur. J. Lipid Sci. Technol. 2018, 120, 1700205. [Google Scholar]
- Garti, N.; Widlak, N.R. Cocoa Butter and Related Compounds; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Biswas, N.; Cheow, Y.; Tan, C.; Siow, L. Blending of palm mid-fraction, refined bleached deodorized palm kernel oil or palm stearin for cocoa butter alternative. J. Am. Oil Chem. Soc. 2016, 93, 1415–1427. [Google Scholar]
- Heinrich, U.; Stahl, W. Chocolate and Skin Health: Effects of Dietary Cocoa Polyphenols. In Chocolate and Health: Chemistry, Nutrition and Therapy; RSC Publishing: Cambridge, UK, 2015; p. 179. [Google Scholar]
- Torres-Moreno, M.; Torrescasana, E.; Salas-Salvadó, J.; Blanch, C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015, 166, 125–132. [Google Scholar] [PubMed]
- Mahjabeen, S.; Hatipoglu, M.K.; Chandra, V.; Benbrook, D.M.; Garcia-Contreras, L. Optimization of a vaginal suppository formulation to deliver SHetA2 as a novel treatment for cervical dysplasia. J. Pharm. Sci. 2018, 107, 638–646. [Google Scholar] [PubMed]
- Kuo, Y.C.; Chao, I.W. Conjugation of melanotransferrin antibody on solid lipid nanoparticles for mediating brain cancer malignancy. Biotechnol. Prog. 2016, 32, 480–490. [Google Scholar] [PubMed]
- Morgan, P.; Maldonado-Codina, C.; Read, M.; Dobson, C. Compositions and Uses and Methods Relating Thereto. U.S. Patent 16/061,606, 27 December 2018. [Google Scholar]
- Sohail, M.; Rakha, A.; Butt, M.S.; Iqbal, M.J.; Rashid, S. Rice bran nutraceutics: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3771–3780. [Google Scholar]
- Wang, Y. Applications of Rice Bran Oil. In Rice Bran and Rice Bran Oil; Cheong, L.-Z., Xu, X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–168. [Google Scholar]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green cosmetic surfactant from rice: Characterization and application. Cosmetics 2015, 2, 322–341. [Google Scholar]
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar]
- Angkuratipakorn, T.; Sriprai, A.; Tantrawong, S.; Chaiyasit, W.; Singkhonrat, J. Fabrication and characterization of rice bran oil-in-water Pickering emulsion stabilized by cellulose nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 310–319. [Google Scholar]
- Pengkumsri, N.; Chaiyasut, C.; Sivamaruthi, B.S.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Chaiyasut, K.; Kesika, P. The influence of extraction methods on composition and antioxidant properties of rice bran oil. Food Sci. Technol. 2015, 35, 493–501. [Google Scholar]
- Eleftheriadis, G.K.; Mantelou, P.; Karavasili, C.; Chatzopoulou, P.; Katsantonis, D.; Irakli, M.; Mygdalia, A.; Vizirianakis, I.S.; Fatouros, D.G. Development and characterization of a self-nanoemulsifying drug delivery system comprised of rice bran oil for poorly soluble drugs. Aaps Pharmscitech 2019, 20, 78. [Google Scholar]
- Rigo, L.A.; da Silva, C.R.; de Oliveira, S.M.; Cabreira, T.N.; da Silva, C.d.B.; Ferreira, J.; Beck, R.C.R. Nanoencapsulation of rice bran oil increases its protective effects against UVB radiation-induced skin injury in mice. Eur. J. Pharm. Biopharm. 2015, 93, 11–17. [Google Scholar]
- Hutchinson, J.H.; Seiders, T.J.; Swaney, J.S. Lysophosphatidic Acid Receptor Antagonists for the Treatment of Conditions or Diseases of the Eye. U.S. Patent 13/704,276, 26 September 2013. [Google Scholar]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar]
- Lee, J.; Kim, J.; Kim, S.; Min, D.-H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [PubMed]
- Yadav, I.; Nayak, S.K.; Rathnam, V.S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing effect of graphene oxide reinforcement on the properties of poly (vinyl alcohol) and carboxymethyl tamarind gum based phase-separated film. J. Mech. Behav. Biomed. Mater. 2018, 81, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S. A combined self-assembly and calcination method for preparation of nanoparticles-assembled cobalt oxide nanosheets using graphene oxide as template and their application for non-enzymatic glucose biosensing. J. Colloid Interface Sci. 2017, 485, 159–166. [Google Scholar] [PubMed]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar]
- Mehra, N.K.; Cai, D.; Kuo, L.; Hein, T.; Palakurthi, S. Safety and toxicity of nanomaterials for ocular drug delivery applications. Nanotoxicology 2016, 10, 836–860. [Google Scholar]
- Dao, T.D.; Jeong, H.M. Novel stearic acid/graphene core–shell composite microcapsule as a phase change material exhibiting high shape stability and performance. Sol. Energy Mater. Sol. Cells 2015, 137, 227–234. [Google Scholar]
- Svanberg, L.; Ahrné, L.; Lorén, N.; Windhab, E. Effect of sugar, cocoa particles and lecithin on cocoa butter crystallisation in seeded and non-seeded chocolate model systems. J. Food Eng. 2011, 104, 70–80. [Google Scholar]
- Liu, H.; Cheng, J.; Chen, F.; Hou, F.; Bai, D.; Xi, P.; Zeng, Z. Biomimetic and cell-mediated mineralization of hydroxyapatite by carrageenan functionalized graphene oxide. ACS Appl. Mater. Interfaces 2014, 6, 3132–3140. [Google Scholar]
- Zúñiga-Diaz, J.; Reyes-Dorantes, E.; Quinto-Hernandez, A.; Porcayo-Calderon, J.; Gonzalez-Rodriguez, J.; Martinez-Gomez, L. Oil extraction from “Morelos Rice” bran: Kinetics and raw oil stability. J. Chem. 2017, 2017, 3837506. [Google Scholar]
- Suparman, S. The use of Fourier transform infrared spectroscopy (FTIR) and gas chromatography mass spectroscopy (GCMS) for halal authentication in imported chocolate with various variants. J. Food Pharm. Sci. 2015, 3, 6–11. [Google Scholar]
- Antony, B.; Sharma, S.; Mehta, B.M.; Ratnam, K.; Aparnathi, K. Study on FT-MIR spectra of ghee (anhydrous milk fat). Br. Food J. 2017, 119, 181–189. [Google Scholar]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C.; Naghavi, M.S.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar]
- Vuppaladadium, S.S.R.; Agarwal, T.; Kulanthaivel, S.; Mohanty, B.; Barik, C.S.; Maiti, T.K.; Pal, S.; Pal, K.; Banerjee, I. Silanization improves biocompatibility of graphene oxide. Mater. Sci. Eng. C 2020, 110, 110647. [Google Scholar]
- Gascho, J.L.; Costa, S.F.; Recco, A.A.; Pezzin, S.H. Graphene oxide films obtained by vacuum filtration: X-ray diffraction evidence of crystalline reorganization. J. Nanomater. 2019, 2019, 5963148. [Google Scholar]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar]
- Pouraliakbar, H.; Jandaghi, M.R.; Baygi, S.J.M.; Khalaj, G. Microanalysis of crystallographic characteristics and structural transformations in SPDed AlMnSi alloy by dual-straining. J. Alloys Compd. 2017, 696, 1189–1198. [Google Scholar]
- Mimouni, R.; Kamoun, O.; Yumak, A.; Mhamdi, A.; Boubaker, K.; Petkova, P.; Amlouk, M. Effect of Mn content on structural, optical, opto-thermal and electrical properties of ZnO: Mn sprayed thin films compounds. J. Alloys Compd. 2015, 645, 100–111. [Google Scholar]
- Le Révérend, B.J.; Fryer, P.J.; Coles, S.; Bakalis, S. A method to qualify and quantify the crystalline state of cocoa butter in industrial chocolate. J. Am. Oil Chem. Soc. 2010, 87, 239–246. [Google Scholar]
- Moja, T.; Mishra, A.; Mishra, S. Nano Size Magnetite Particles Layered with the Blend of Conductive Polymer and Superadsorbent Hydrogel: A Core–Shell Based Nanocomposite for Trivalent Arsenide Uptake form Aqueous Solution. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2131–2142. [Google Scholar]
- Risan, J.; Jain, G.; Pendola, M.; Evans, J.S. Intracrystalline incorporation of nacre protein hydrogels modifies the mechanical properties of calcite crystals: A microcompression study. J. Mater. Chem. B 2018, 6, 4191–4196. [Google Scholar] [PubMed]
- Galbraith, S.C.; Flood, A.E.; Rugmai, S.; Chirawatkul, P. Relationship between Surface Roughness, Internal Crystal Perfection, and Crystal Growth Rate. Chem. Eng. Technol. 2016, 39, 199–207. [Google Scholar]
- Uvanesh, K.; Sagiri, S.S.; Banerjee, I.; Shaikh, H.; Pramanik, K.; Anis, A.; Pal, K. Effect of tween 20 on the properties of stearate oleogels: An in-depth analysis. J. Am. Oil Chem. Soc. 2016, 93, 711–719. [Google Scholar]
- Hopper, R.; Uhlmann, D. Solute redistribution during crystallization at constant velocity and constant temperature. J. Cryst. Growth 1974, 21, 203–213. [Google Scholar]
- Soto, P.L.; Hiranita, T.; Xu, M.; Hursh, S.R.; Grandy, D.K.; Katz, J.L. Dopamine D 2-like receptors and behavioral economics of food reinforcement. Neuropsychopharmacology 2016, 41, 971. [Google Scholar] [PubMed] [Green Version]
- Sagiri, S.S.; Sharma, V.; Basak, P.; Pal, K. Mango butter emulsion gels as cocoa butter equivalents: Physical, thermal, and mechanical analyses. J. Agric. Food Chem. 2014, 62, 11357–11368. [Google Scholar] [CrossRef] [PubMed]
- Neto, A.J.P.; Fileti, E.E. Elucidating the amphiphilic character of graphene oxide. Phys. Chem. Chem. Phys. 2018, 20, 9507–9515. [Google Scholar]
- Toussaint, J.-F.O.; Southern, J.F.; Fuster, V.; Kantor, H.L. Water diffusion properties of human atherosclerosis and thrombosis measured by pulse field gradient nuclear magnetic resonance. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 542–546. [Google Scholar]
- Loisel, C.; Keller, G.; Lecq, G.; Bourgaux, C.; Ollivon, M. Phase transitions and polymorphism of cocoa butter. J. Am. Oil Chem. Soc. 1998, 75, 425–439. [Google Scholar]
- Knez, Z.; Weidner, E. Precipitation of solids with dense gases. Ind. Chem. Libr. 2001, 9, 587–611. [Google Scholar]
- Gutiérrez, T.J. State-of-the-Art Chocolate Manufacture: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1313–1344. [Google Scholar]
- Tobolsky, A.V. Stress relaxation studies of the viscoelastic properties of polymers. J. Appl. Phys. 1956, 27, 673–685. [Google Scholar]
- Singh, V.; Pal, K.; Banerjee, I.; Pramanik, K.; Anis, A.; Al-Zahrani, S. Novel organogel based lyotropic liquid crystal physical gels for controlled delivery applications. Eur. Polym. J. 2015, 68, 326–337. [Google Scholar]
- Sagiri, S.S.; Singh, V.K.; Kulanthaivel, S.; Banerjee, I.; Basak, P.; Battachrya, M.; Pal, K. Stearate organogel–gelatin hydrogel based bigels: Physicochemical, thermal, mechanical characterizations and in vitro drug delivery applications. J. Mech. Behav. Biomed. Mater. 2015, 43, 1–17. [Google Scholar] [PubMed]
- Uvanesh, K.; Nayak, S.K.; Sagiri, S.S.; Banerjee, I.; Ray, S.S.; Pal, K. Effect of Non-Ionic Hydrophilic and Hydrophobic Surfactants on the Properties on the Stearate Oleogels: A Comparative Study. In Nutraceuticals and Innovative Food Products for Healthy Living and Preventive Care; IGI Global: Hershey, PA, USA, 2018; pp. 260–279. [Google Scholar]
- Sagiri, S.S.; Kumar, U.; Champaty, B.; Singh, V.K.; Pal, K. Thermal, electrical, and mechanical properties of tween 80/span 80–based organogels and its application in iontophoretic drug delivery. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Karaman, S.; Yilmaz, M.T.; Toker, O.S.; Dogan, M. Stress relaxation/creep compliance behaviour of kashar cheese: Scanning electron microscopy observations. Int. J. Dairy Technol. 2016, 69, 254–261. [Google Scholar]
- Herak, D.; Kabutey, A.; Choteborsky, R.; Petru, M.; Sigalingging, R. Mathematical models describing the relaxation behaviour of Jatropha curcas L. bulk seeds under axial compression. Biosyst. Eng. 2015, 131, 77–83. [Google Scholar]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control Release 1987, 5, 23–36. [Google Scholar]
- Guragain, S.; Torad, N.L.; Alghamdi, Y.G.; Alshehri, A.A.; Kim, J.; Bastakoti, B.P.; Yamauchi, Y. Synthesis of nanoporous calcium carbonate spheres using double hydrophilic block copolymer poly (acrylic acid-bN-isopropylacrylamide). Mater. Lett. 2018, 230, 143–147. [Google Scholar]
- Soares, P.I.; Sousa, A.I.; Silva, J.C.; Ferreira, I.M.; Novo, C.M.; Borges, J.P. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016, 147, 304–312. [Google Scholar]
- Avachat, A.; Kotwal, V. Design and evaluation of matrix-based controlled release tablets of diclofenac sodium and chondroitin sulphate. Aaps Pharmscitech 2007, 8, 51–56. [Google Scholar]
- Shah, K.U.; Khan, G.M. Regulating drug release behavior and kinetics from matrix tablets based on fine particle-sized ethyl cellulose ether derivatives: An in vitro and in vivo evaluation. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.D.; Liu, W.; Selomulya, C.; Chen, X.D. On spray drying of uniform silica-based microencapsulates for controlled release. Soft Matter 2011, 7, 11416–11424. [Google Scholar]
- Jeong, Y.-I.; Na, H.-S.; Seo, D.-H.; Kim, D.-G.; Lee, H.-C.; Jang, M.-K.; Na, S.-K.; Roh, S.-H.; Kim, S.-I.; Nah, J.-W. Ciprofloxacin-encapsulated poly (DL-lactide-co-glycolide) nanoparticles and its antibacterial activity. Int. J. Pharm. 2008, 352, 317–323. [Google Scholar]
- Black, M.T.; Stachyra, T.; Platel, D.; Girard, A.-M.; Claudon, M.; Bruneau, J.-M.; Miossec, C. Mechanism of action of the antibiotic NXL101, a novel nonfluoroquinolone inhibitor of bacterial type II topoisomerases. Antimicrob. Agents Chemother. 2008, 52, 3339–3349. [Google Scholar]
- Da Silva, A.D.; De Almeida, M.V.; De Souza, M.V.; Couri, M.R. Biological activity and synthetic metodologies for the preparation of fluoroquinolones, a class of potent antibacterial agents. Curr. Med. Chem. 2003, 10, 21–39. [Google Scholar]
- Sheikholeslami, P.; Muirhead, B.; Baek, D.S.H.; Wang, H.; Zhao, X.; Sivakumaran, D.; Boyd, S.; Sheardown, H.; Hoare, T. Hydrophobically-modified poly (vinyl pyrrolidone) as a physically-associative, shear-responsive ophthalmic hydrogel. Exp. Eye Res. 2015, 137, 18–31. [Google Scholar]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar]










| Formulations | Peaks | Peak Position (°2θ) | FWHM (°2θ) | d-Spacing (Å) | Crystallite Size (nm) | Lattice Strain |
|---|---|---|---|---|---|---|
| CR1 | Peak 3 | 24.201 | 1.238 | 4.267 | 7.960 | 0.025 |
| Peak 4 | 25.269 | 0.585 | 4.089 | 16.890 | 0.011 | |
| Peak 5 | 25.979 | 4.541 | 3.979 | 2.180 | 0.086 | |
| Average | 2.122 | 4.112 | 9.010 | 0.041 | ||
| CR2 | Peak 3 | 21.222 | 4.565 | 4.857 | 2.150 | 0.106 |
| Peak 4 | 22.564 | 2.670 | 4.572 | 3.680 | 0.058 | |
| Peak 5 | 23.743 | 1.166 | 4.348 | 8.450 | 0.024 | |
| Average | 2.800 | 4.592 | 4.760 | 0.063 | ||
| CR3 | Peak 3 | 22.253 | 6.775 | 4.635 | 1.450 | 0.150 |
| Peak 4 | 22.253 | 1.978 | 4.635 | 4.970 | 0.044 | |
| Peak 5 | 23.441 | 0.930 | 4.403 | 10.590 | 0.020 | |
| Average | 3.228 | 4.558 | 5.670 | 0.071 | ||
| CR4 | Peak 3 | 22.659 | 5.259 | 4.553 | 1.870 | 0.115 |
| Peak 4 | 23.598 | 0.710 | 4.375 | 13.870 | 0.015 | |
| Peak 5 | 24.700 | 0.679 | 4.182 | 14.530 | 0.014 | |
| Average | 2.216 | 4.370 | 10.090 | 0.048 | ||
| CR5 | Peak 3 | 23.341 | 4.670 | 4.422 | 2.110 | 0.099 |
| Peak 4 | 24.046 | 0.679 | 4.294 | 14.520 | 0.014 | |
| Peak 5 | 25.139 | 0.559 | 4.110 | 17.670 | 0.011 | |
| Average | 1.969 | 4.275 | 11.433 | 0.041 |
| Parameters | Formulations | ||||
|---|---|---|---|---|---|
| CR1 | CR2 | CR3 | CR4 | CR5 | |
| a | 35.000 | 35.000 | 35.000 | 35.000 | 35.000 |
| k (°C/m·s) | −0.95 | −0.82 | −0.74 | −0.89 | −0.87 |
| R2 | 0.995 | 0.998 | 0.998 | 0.994 | 0.998 |
| Peaks | Peak Position (Temperature, °C) | ||||
|---|---|---|---|---|---|
| CR1 | CR2 | CR3 | CR4 | CR5 | |
| Peak 1 (Red arrow) | 19.71 | 21.27 | 21.28 | 19.73 | 20.75 |
| Peak 2 (green arrow) | 26.84 | 27.36 | 27.86 | 26.35 | 27.36 |
| Peak 3 (Major peak) | 30.88 | 30.88 | 30.89 | 30.89 | 30.38 |
| Study | Model | Parameters | CR1D | CR2D | CR3D | CR4D | CR5D |
|---|---|---|---|---|---|---|---|
| Diffusion | Korsmeyer-Peppas | K | 0.064 | 0.127 | 0.167 | 0.076 | 0.142 |
| n | 0.537 | 0.391 | 0.337 | 0.401 | 0.271 | ||
| R2 | 0.994 | 0.999 | 0.999 | 0.994 | 0.997 | ||
| Peppas-Sahlin | Kd | 0.001 | 0.068 | 0.001 | 0.073 | 0.076 | |
| Kr | 0.064 | 0.070 | 0.165 | 0.013 | 0.073 | ||
| m | 0.268 | 0.228 | 0.169 | 0.293 | 0.165 | ||
| Kd/Kr | 0.016 | 0.975 | 0.004 | 5.474 | 1.045 | ||
| R2 | 0.994 | 0.999 | 0.999 | 0.995 | 0.998 | ||
| Corneal permeation | Korsmeyer-Peppas | K | 0.086 | 0.159 | 0.108 | 0.402 | 0.362 |
| n | 0.353 | 0.241 | 0.368 | 0.125 | 0.144 | ||
| R2 | 0.998 | 0.998 | 0.999 | 0.999 | 0.999 | ||
| Peppas-Sahlin | Kd | 0.062 | 0.128 | 0.007 | 0.394 | 0.270 | |
| Kr | 0.032 | 0.038 | 0.101 | 0.0002 | 0.097 | ||
| m | 0.226 | 0.176 | 0.188 | 0.130 | 0.107 | ||
| Kd/Kr | 1.904 | 3.334 | 0.066 | 1350.906 | 2.779 | ||
| R2 | 0.999 | 0.998 | 0.999 | 0.999 | 0.999 |
| Formulations | CB (g) | RBO (g) | GO | CPH (mg) | |
|---|---|---|---|---|---|
| (mg) | (wt%) | ||||
| CR1 | 3.5 | 6.5 | 0.0 | 0.000 | 00 |
| CR2 | 3.5 | 6.5 | 0.5 | 0.005 | 00 |
| CR3 | 3.5 | 6.5 | 1.5 | 0.015 | 00 |
| CR4 | 3.5 | 6.5 | 2.5 | 0.025 | 00 |
| CR5 | 3.5 | 6.5 | 5.0 | 0.050 | 00 |
| CR1D | 3.5 | 6.5 | 0.0 | 0.000 | 50 |
| CR2D | 3.5 | 6.5 | 0.5 | 0.005 | 50 |
| CR3D | 3.5 | 6.5 | 1.5 | 0.015 | 50 |
| CR4D | 3.5 | 6.5 | 2.5 | 0.025 | 50 |
| CR5D | 3.5 | 6.5 | 5.0 | 0.050 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, D.; Choudhary, B.; Mohanty, B.; Sarkar, P.; Anis, A.; Cerqueira, M.A.; Banerjee, I.; Maji, S.; Pal, K. Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels. Gels 2020, 6, 43. https://doi.org/10.3390/gels6040043
Qureshi D, Choudhary B, Mohanty B, Sarkar P, Anis A, Cerqueira MA, Banerjee I, Maji S, Pal K. Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels. Gels. 2020; 6(4):43. https://doi.org/10.3390/gels6040043
Chicago/Turabian StyleQureshi, Dilshad, Barbiee Choudhary, Biswaranjan Mohanty, Preetam Sarkar, Arfat Anis, Miguel A. Cerqueira, Indranil Banerjee, Samarendra Maji, and Kunal Pal. 2020. "Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels" Gels 6, no. 4: 43. https://doi.org/10.3390/gels6040043
APA StyleQureshi, D., Choudhary, B., Mohanty, B., Sarkar, P., Anis, A., Cerqueira, M. A., Banerjee, I., Maji, S., & Pal, K. (2020). Graphene Oxide Increases Corneal Permeation of Ciprofloxacin Hydrochloride from Oleogels: A Study with Cocoa Butter-Based Oleogels. Gels, 6(4), 43. https://doi.org/10.3390/gels6040043