Structural and Technological Approach to Reveal the Role of the Lipid Phase in the Formation of Soy Emulsion Gels with Chia Oil
Abstract
:1. Introduction
2. Results and Discussion
2.1. Proximate Composition
2.2. Syneresis and pH Values
2.3. Color Measurement
2.4. Penetration Test
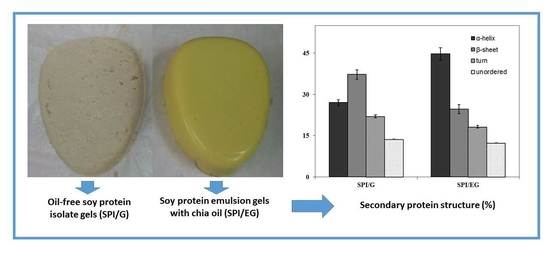
2.5. FT-Raman Spectroscopic Analysis
Protein Secondary Structure
3. Conclusions
4. Materials and Methods
4.1. Sample Preparation
4.2. Proximate Composition Analysis
4.3. Syneresis and pH Values
4.4. Color Measurement
4.5. Penetration Test
4.6. FT-Raman Spectroscopic Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Mao, L.; Hou, Z.; Miao, S.; Gao, Y. Development of Emulsion Gels for the Delivery of Functional Food Ingredients: From Structure to Functionality. Food Eng. Rev. 2019, 11, 245–258. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. Preparation, structure-property relationships and applications of different emulsion gels: Bulk emulsion gels, emulsion gel particles, and fluid emulsion gels. Trends Food Sci. Technol. 2020, 102, 123–137. [Google Scholar] [CrossRef]
- Paglarini, C.D.S.; Vidal, V.A.S.; Martini, S.; Cunha, R.L.; Pollonio, M.A.R. Protein-based hydrogelled emulsions and their application as fat replacers in meat products: A review. Crit. Rev. Food Sci. 2020. [Google Scholar] [CrossRef]
- Herrero, A.M.; Ruiz-Capillas, C. Novel lipid materials based on gelling procedures as fat analogues in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 39, 1–6. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Matsumura, Y.; Sirison, J.; Ishi, T.; Matsumiya, K. Soybean lipophilic proteins—Origin and functional properties as affected by interaction with storage proteins. Curr. Opin. Colloid Interface Sci. 2017, 28, 120–128. [Google Scholar] [CrossRef]
- Tang, C.H. Emulsifying properties of soy proteins: A critical review with emphasis on the role of conformational flexibility. Crit. Rev. Food Sci. 2017, 57, 2636–2679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Daubert, C.R.; Foegeding, E. Fracture analysis of alginate gels. J. Food Sci. 2005, 70, 425–431. [Google Scholar] [CrossRef]
- Roopa, B.S.; Bhattacharya, S. Alginate gels: II. Stability at different processing conditions. J. Food Process Eng. 2010, 33, 466–480. [Google Scholar] [CrossRef]
- Gaonkar, A.G. Surface and interfacial activities and emulsion characteristics of some food hydrocolloids. Food Hydrocoll. 1991, 5, 329–337. [Google Scholar] [CrossRef]
- Herrero, A.M.; Jimenez-Colmenero, F.; Ruiz-Capillas, C. Improving lipid content in muscle-based food: New strategies for developing fat replacers based on gelling processes using healthy edible oils. In Reformulation as a Strategy for Developing Healthier Food Products; Raikos, V., Ranawana, V., Eds.; Springer: Cham, Switzerland, 2019; pp. 185–198. [Google Scholar]
- Dickinson, E. Stabilising emulsion-based colloidal structures with mixed food ingredients. J. Sci. Food Agric. 2013, 93, 710–721. [Google Scholar] [CrossRef]
- Pintado, T.; Ruiz-Capillas, C.; Jimenez-Colmenero, F.; Carmona, P.; Herrero, A.M. Oil-in-water emulsion gels stabilized with chia (Salvia hispanica L.) and cold gelling agents: Technological and infrared spectroscopic characterization. Food Chem. 2015, 185, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Ruiz-Capillas, C.; Pintado, T.; Carmona, P.; Jimenez-Colmenero, F. Elucidation of lipid structural characteristics of chia oil emulsion gels by Raman spectroscopy and their relationship with technological properties. Food Hydrocoll. 2018, 77, 212–219. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A.M. Development of Meat Products with Healthier Lipid Content: Vibrational Spectroscopy. Foods 2021, 10, 341. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M. Raman spectroscopy for monitoring protein structure in muscle food systems. Crit. Rev. Food Sci. 2008, 48, 512–523. [Google Scholar] [CrossRef]
- Howell, N.K.; Herman, H.; Li-Chan, E.C.Y. Elucidation of protein-lipid interactions in a lysozyme-corn oil system by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 2001, 49, 1529–1533. [Google Scholar] [CrossRef]
- LiChan, E.C.Y. The applications of Raman spectroscopy in food science. Trends Food Sci. Technol. 1996, 7, 361–370. [Google Scholar] [CrossRef]
- Muik, B.; Lendl, B.; Molina-Díaz, A.; Ayora-Cañada, M.J. Direct, reagent free determination of free fatty acid content in olive oil and olives by Fourier transform Raman spectrometry. Anal. Chim. Acta 2003, 487, 211–220. [Google Scholar] [CrossRef]
- Meng, G.; Chan, J.C.K.; Rousseau, D.; Li-Chan, E.C.Y. Study of protein-lipid interactions at the bovine serum albumin/oil interface by Raman microspectroscopy. J. Agric. Food Chem. 2005, 53, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Capitani, M.I.; Nolasco, S.M.; Tomas, M.C. Stability of oil-in-water (O/W) emulsions with chia (Salvia hispanica L.) mucilage. Food Hydrocoll. 2016, 61, 537–546. [Google Scholar] [CrossRef]
- Ayerza, R.; Coates, W. Ground chia seed and chia oil effects on plasma lipids and fatty acids in the rat. Nutr. Res. 2005, 25, 995–1003. [Google Scholar] [CrossRef]
- Pintado, T.; Herrero, A.M.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Emulsion gels as potential fat replacers delivering β-glucan and healthy lipid content for food applications. J. Food Sci. Technol. 2016, 53, 4336–4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-González, I.; Merino-Álvarez, E.; Salvador, M.; Pintado, T.; Ruiz-Capillas, C.; Jiménez-Colmenero, F.; Herrero, A.M. Chia (Salvia hispanica L.) a promising alternative for conventional and gelled emulsions: Technological and lipid structural characteristics. Gels 2019, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Colmenero, F.; Cofrades, S.; Herrero, A.M.; Fernández-Martín, F.; Rodríguez-Salas, L.; Ruiz-Capillas, C. Konjac gel fat analogue for use in meat products: Comparison with pork fats. Food Hydrocoll. 2012, 26, 63–72. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Herrero, A.; Pintado, T.; Solas, M.T.; Ruiz-Capillas, C. Influence of emulsified olive oil stabilizing system used for pork backfat replacement in frankfurters. Food Res. Int. 2010, 43, 2068–2076. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Ruiz-Capillas, C.; Salvador, M.; Herrero, A.M. Emulsion gels as delivery systems for phenolic compounds: Nutritional, technological and structural properties. Food Chem. 2021, 339, 128049. [Google Scholar] [CrossRef]
- Imran, M.; Nadeem, M.; Manzoor, M.F.; Javed, A.; Ali, Z.; Akhtar, M.N.; Ali, M.; Hussain, Y. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 162. [Google Scholar] [CrossRef] [Green Version]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.K.; Nolasco, S.M.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Compos. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Dickinson, E.; Chen, J. Heat-set whey protein emulsion gels: Role of active and inactive filler particles. J. Disper. Sci. Technol. 1999, 20, 197–213. [Google Scholar] [CrossRef]
- Geremias-Andrade, I.M.; Souki, N.P.D.B.G.; Moraes, I.C.F.; Pinho, S.C. Rheological and mechanical characterization of curcumin-loaded emulsion-filled gels produced with whey protein isolate and xanthan gum. LWT Food Sci. Technol. 2017, 86, 166–173. [Google Scholar] [CrossRef]
- Herrero, A.M.; Carmona, P.; Pintado, T.; Jiménez-Colmenero, F.; Ruíz-Capillas, C. Infrared spectroscopic analysis of structural features and interactions in olive oil-in-water emulsions stabilized with soy protein. Food Res. Int. 2011, 44, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lefévre, T.; Subirade, M.; Paquin, P. Changes and roles of secondary structures of whey protein for the formation of protein membrane at soy oil/water interface under high-pressure homogenization. J. Agric. Food Chem. 2007, 55, 10924–10931. [Google Scholar] [CrossRef]
- Alix, A.J.P.; Pedanou, G.; Berjot, M. Fast determination of the quantitative secondary structure of proteins by using some parameters of the Raman amide I band. J. Mol. Struct. 1988, 174, 159–164. [Google Scholar] [CrossRef]
- Niu, F.; Niu, D.; Zhang, H.; Chang, C.; Gu, L.; Su, Y.; Yang, Y. Ovalbumin/gum Arabic-stabilized emulsion: Rheology, emulsion characteristics, and Raman spectroscopic study. Food Hydrocoll. 2016, 52, 607–614. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis; Association of Official Analytical Chemistry: Rockville, MD, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]



| Samples * | SPI | Gelling Agent | Water | Chia Oil | ||
|---|---|---|---|---|---|---|
| Sodium Alginate | CaSO4 | Sodium Pyrophosphate | ||||
| SPI/G | 23.25 | 1 | 1 | 0.75 | 74 | |
| SPI/EG | 23.25 | 1 | 1 | 0.75 | 34 | 40 |
| Parameters | Samples * | |
|---|---|---|
| SPI/G | SPI/EG | |
| pH | 7.26 ± 0.04 a | 7.31 ± 0.03 a |
| L* | 64.77 ± 0.87 b | 74.61 ± 0.81 a |
| a* | 3.14 ± 0.32 a | 1.52 ± 0.20 b |
| b* | 18.56 ± 0.41 b | 19.45 ± 0.23 a |
| PF | 1.21 ± 0.33 b | 1.87 ± 0.04 a |
| GS | 10.40 ± 0.86 b | 17.93 ± 0.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero, A.M.; Ruiz-Capillas, C. Structural and Technological Approach to Reveal the Role of the Lipid Phase in the Formation of Soy Emulsion Gels with Chia Oil. Gels 2021, 7, 48. https://doi.org/10.3390/gels7020048
Herrero AM, Ruiz-Capillas C. Structural and Technological Approach to Reveal the Role of the Lipid Phase in the Formation of Soy Emulsion Gels with Chia Oil. Gels. 2021; 7(2):48. https://doi.org/10.3390/gels7020048
Chicago/Turabian StyleHerrero, Ana M., and Claudia Ruiz-Capillas. 2021. "Structural and Technological Approach to Reveal the Role of the Lipid Phase in the Formation of Soy Emulsion Gels with Chia Oil" Gels 7, no. 2: 48. https://doi.org/10.3390/gels7020048
APA StyleHerrero, A. M., & Ruiz-Capillas, C. (2021). Structural and Technological Approach to Reveal the Role of the Lipid Phase in the Formation of Soy Emulsion Gels with Chia Oil. Gels, 7(2), 48. https://doi.org/10.3390/gels7020048