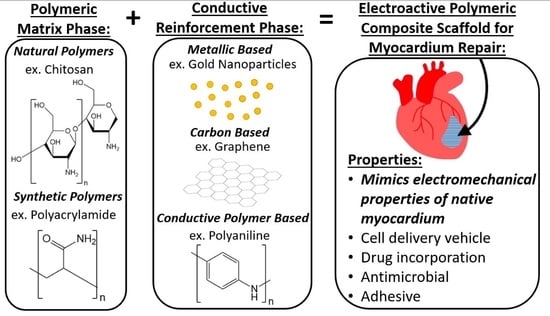
Electroactive Polymeric Composites to Mimic the Electromechanical Properties of Myocardium in Cardiac Tissue Repair
Abstract
:1. Introduction
2. A Composite Approach to Biomimicry for Cardiac Tissue Engineering
3. Reinforcement Methods to Mimic the Electromechanical Properties of Myocardium with Polymeric Composites
3.1. Overview of Conductive Reinforcement Types
3.2. Metallic-Based Reinforcement
3.3. Carbon-Based Reinforcement
3.4. Conductive Polymer-Based Reinforcement
4. Future Outlook
5. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology/American College of Cardiology/American Heart Association/World Heart Federation Task Force for the Universal Definition of Myocardial, I. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Poss, K.D. Cardiac regenerative capacity and mechanisms. Annu. Rev. Cell Dev. Biol. 2012, 28, 719–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CDC (Centers for Disease Control and Prevention). Heart Disease in the United States. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 12 December 2020).
- Ventricular Assist Device. Available online: https://www.mayoclinic.org/tests-procedures/ventricular-assist-device/about/pac-20384529 (accessed on 12 December 2020).
- Baei, P.; Hosseini, M.; Baharvand, H.; Pahlavan, S. Electrically conductive materials for in vitro cardiac microtissue engineering. J. Biomed. Mater. Res. A 2020, 108, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Russell, B. Stem cell therapy for cardiac repair. J. Cardiovasc. Nurs. 2009, 24, 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolli, R.; Ghafghazi, S. Stem cells: Cell therapy for cardiac repair: What is needed to move forward? Nat. Rev. Cardiol. 2017, 14, 257–258. [Google Scholar] [CrossRef]
- Zhang, J. Engineered Tissue Patch for Cardiac Cell Therapy. Curr. Treat Options Cardiovasc. Med. 2015, 17, 399. [Google Scholar] [CrossRef] [Green Version]
- Qazi, T.H.; Rai, R.; Dippold, D.; Roether, J.E.; Schubert, D.W.; Rosellini, E.; Barbani, N.; Boccaccini, A.R. Development and characterization of novel electrically conductive PANI-PGS composites for cardiac tissue engineering applications. Acta. Biomater. 2014, 10, 2434–2445. [Google Scholar] [CrossRef]
- Li, J. An anti-oxidative and conductive composite scaffold for cardiac tissue engineering. Composites 2020, 199, 1203–1213. [Google Scholar]
- Bhana, B.; Iyer, R.K.; Chen, W.L.; Zhao, R.; Sider, K.L.; Likhitpanichkul, M.; Simmons, C.A.; Radisic, M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010, 105, 1148–1160. [Google Scholar] [CrossRef]
- Tallawi, M.; Rai, R.; Boccaccini, A.R.; Aifantis, K.E. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng. Part. B. Rev. 2015, 21, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reis, L.A.; Chiu, L.L.; Feric, N.; Fu, L.; Radisic, M. Biomaterials in myocardial tissue engineering. J. Tissue Eng. Regen. Med. 2016, 10, 11–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palza, H.; Zapata, P.A.; Angulo-Pineda, C. Electroactive Smart Polymers for Biomedical Applications. Materials 2019, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheffield, C.; Meyers, K.; Johnson, E.; Rajachar, R. Application of Composite Hydrogels to Control Physical Properties in Tissue Engineering and Regenerative Medicine. Gels 2018, 4, 51. [Google Scholar] [CrossRef] [Green Version]
- Matthews, F.L. Composite Materials: Engineering and Science; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Baei, P.; Jalili-Firoozinezhad, S.; Rajabi-Zeleti, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically conductive gold nanoparticle-chitosan thermosensitive hydrogels for cardiac tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 63, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Nazari, H. Nanofibrous composites reinforced by MoS2 Nanosheets as a conductive scaffold for cardiac tissue engineering. Chem. Select 2019, 4, 11557–11563. [Google Scholar]
- You, J.O.; Rafat, M.; Ye, G.J.; Auguste, D.T. Nanoengineering the heart: Conductive scaffolds enhance connexin 43 expression. Nano Lett. 2011, 11, 3643–3648. [Google Scholar] [CrossRef]
- Hosoyama, K. Multi-functional thermo-crosslinkable collagen-metal nanoparticle composites for tissue regeneration: Nanosilver vs. nanogold. RSC Adv. 2017, 7, 47704–47708. [Google Scholar] [CrossRef] [Green Version]
- Jo, H.; Sim, M.; Kim, S.; Yang, S.; Yoo, Y.; Park, J.H.; Yoon, T.H.; Kim, M.G.; Lee, J.Y. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017, 48, 100–109. [Google Scholar] [CrossRef]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Chen, D.; Wang, Z.; Zhang, Z.; Xia, Y.; Xue, H.; Liu, Y. Preparation of an Electrically Conductive Graphene Oxide/Chitosan Scaffold for Cardiac Tissue Engineering. Appl. Biochem. Biotechnol. 2019, 188, 952–964. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nazari, H. Fabrication of graphene-silver/polyurethane nanofibrous scaffolds for cardiac tissue engineering. Polym. Adv. Technol. 2019, 30, 2086–2099. [Google Scholar] [CrossRef]
- Jing, X. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Tsui, J.H.; Ostrovsky-Snider, N.A.; Yama, D.M.P.; Donohue, J.D.; Choi, J.S.; Chavanachat, R.; Larson, J.D.; Murphy, A.R.; Kim, D.H. Conductive Silk-Polypyrrole Composite Scaffolds with Bioinspired Nanotopographic Cues for Cardiac Tissue Engineering. J. Mater. Chem. B 2018, 6, 7185–7196. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, B.; Chen, J.; Bao, R.; Zhang, X.; Liang, S.; Shang, Y.; Liang, W.; Cui, Y.; Fan, G.; et al. An injectable conductive hydrogel encapsulating plasmid DNA-eNOs and ADSCs for treating myocardial infarction. Biomaterials 2018, 160, 69–81. [Google Scholar] [CrossRef]
- Zanjanizadeh Ezazi, N.; Ajdary, R.; Correia, A.; Makila, E.; Salonen, J.; Kemell, M.; Hirvonen, J.; Rojas, O.J.; Ruskoaho, H.J.; Santos, H.A. Fabrication and Characterization of Drug-Loaded Conductive Poly(glycerol sebacate)/Nanoparticle-Based Composite Patch for Myocardial Infarction Applications. ACS Appl. Mater. Interfaces 2020, 12, 6899–6909. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.Y.; Iordachescu, M.; Huang, J.; Hennebert, E.; Kim, S.; Rho, S.; Foo, M.; Flammang, P.; Zeng, H.; Hwang, D.; et al. Sugary interfaces mitigate contact damage where stiff meets soft. Nat. Commun. 2016, 7, 11923. [Google Scholar] [CrossRef]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and Antibiotic, New Facts to an Old Story. Antibiotics 2018, 7. [Google Scholar] [CrossRef] [Green Version]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front Pharmacol. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Smart, S. The biocompatibility of carbon nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, C.; Zhou, Z.; Tan, G.; Zhu, Y.; Mao, C. Electroactive polymers for tissue regeneration: Developments and perspectives. Prog. Polym. Sci. 2018, 81, 144–162. [Google Scholar] [CrossRef]
- Kinnunen, S.M.; Tolli, M.; Valimaki, M.J.; Gao, E.; Szabo, Z.; Rysa, J.; Ferreira, M.P.A.; Ohukainen, P.; Serpi, R.; Correia, A.; et al. Cardiac Actions of a Small Molecule Inhibitor Targeting GATA4-NKX2-5 Interaction. Sci. Rep. 2018, 8, 4611. [Google Scholar] [CrossRef] [Green Version]
- Fidanovski, K.; Mawad, D. Conjugated Polymers in Bioelectronics: Addressing the Interface Challenge. Adv. Healthc. Mater. 2019, 8, e1900053. [Google Scholar] [CrossRef]
- Wu, T.; Cui, C.; Huang, Y.; Liu, Y.; Fan, C.; Han, X.; Yang, Y.; Xu, Z.; Liu, B.; Fan, G.; et al. Coadministration of an Adhesive Conductive Hydrogel Patch and an Injectable Hydrogel to Treat Myocardial Infarction. ACS Appl. Mater. Interfaces 2020, 12, 2039–2048. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable chitosan/kappa-carrageenan hydrogel designed with au nanoparticles: A conductive scaffold for tissue engineering demands. Int. J. Biol. Macromol. 2019, 126, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Pinnaratip, R.; Bhuiyan, M.S.A.; Meyers, K.; Rajachar, R.M.; Lee, B.P. Multifunctional Biomedical Adhesives. Adv. Healthc. Mater. 2019, 8, e1801568. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shi, H.; Moore, S.; Wang, C.; Ash-Shakoor, A.; Mather, P.T.; Henderson, J.H.; Ma, Z. Progressive Myofibril Reorganization of Human Cardiomyocytes on a Dynamic Nanotopographic Substrate. ACS Appl. Mater. Interfaces 2020, 12, 21450–21462. [Google Scholar] [CrossRef] [PubMed]




| Reinforcement Type | Conductive Composite | Conductivity (S/cm) | Modulus (Pa) | Reference |
|---|---|---|---|---|
| Metallic-Based | Chitosan/gold nanoparticle (CS-GNP) hydrogels | 8–13 | 6.1 × 103– 6.8 × 103 | [19] |
| Nylon/molybdenum disulfide (MoS2) nanosheets | 20 × 10−6 | 3 × 106 | [20] | |
| Gold nanoparticles in thiol-HEMA/HEMA scaffolds | 1060–1530 | 0.6 × 106 | [21] | |
| Collagen–silver/gold nanoparticle matrices | 0.75 × 10−4 | N/A | [22] | |
| Carbon-Based | Reduced graphene oxide/polyacrylamide r(GO/PAAm) hydrogel | 1.3 × 10−4 | 50 × 103 | [23] |
| Doped carbon nanofibers in chitosan | 4 | 28.1 × 103 | [24] | |
| Graphene oxide/chitosan (GO/CS) scaffolds | 13.4 | N/A | [25] | |
| Polyvinyl alcohol/chitosan/carbon nanofibers (PVA-CS-CNT) | 3.4 × 10−6 | 130 × 106 | [26] | |
| Reduced graphene oxide-silver (rGO-Ag) nanocomposites in polyurethane (PU) nanofibers | 100 × 10−6 | 210 × 106 | [27] | |
| Chitosan/dopamine/graphene oxide (CS-DA-GO) composite hydrogels | 1.22 × 10−3 | N/A | [28] | |
| Conductive Polymer-Based | Polyaniline (PANI) -poly(glycerol-sebacate) (PGS) composite doped with camphorsulfonic acid | 0.018 | 6 × 106 | [10] |
| Acid-modified silk fibroin–poly (pyrrole) (AMSF + PPy) substrates | 1 | 7 × 106–200 × 106 | [29] | |
| Gelatin/aniline pentamer-glutathione composite (Gel/AP-GSH) | 3.4 × 10−5–1 × 10−4 | 55.1 × 103–142.7 × 103 | [11] | |
| Nitric oxide inducing tetraaniline-polyethylene glycol diacrylate (TA-PEG) and thiolated hyaluronic acid (HA-SH) hydrogel | 2.32 × 10−4 | 23 | [30] | |
| 3i-1000 loaded poly(glycerol sebacate) (PGS)/collagen/ carbonized porous silicon nanoparticle composites | 0.06 | 0.08 × 106 | [31] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyers, K.; Lee, B.P.; Rajachar, R.M. Electroactive Polymeric Composites to Mimic the Electromechanical Properties of Myocardium in Cardiac Tissue Repair. Gels 2021, 7, 53. https://doi.org/10.3390/gels7020053
Meyers K, Lee BP, Rajachar RM. Electroactive Polymeric Composites to Mimic the Electromechanical Properties of Myocardium in Cardiac Tissue Repair. Gels. 2021; 7(2):53. https://doi.org/10.3390/gels7020053
Chicago/Turabian StyleMeyers, Kaylee, Bruce P. Lee, and Rupak M. Rajachar. 2021. "Electroactive Polymeric Composites to Mimic the Electromechanical Properties of Myocardium in Cardiac Tissue Repair" Gels 7, no. 2: 53. https://doi.org/10.3390/gels7020053
APA StyleMeyers, K., Lee, B. P., & Rajachar, R. M. (2021). Electroactive Polymeric Composites to Mimic the Electromechanical Properties of Myocardium in Cardiac Tissue Repair. Gels, 7(2), 53. https://doi.org/10.3390/gels7020053