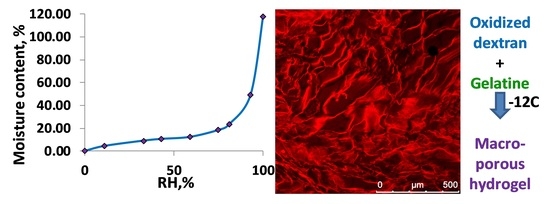
Water Uptake as a Crucial Factor on the Properties of Cryogels of Gelatine Cross-Linked by Dextran Dialdehyde
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Cryogels
4.3. Confocal Laser Scanning Microscopy (CLSM) Images of Cryogels
4.4. Water Vapour Sorption Methods
4.5. Thermogravimetric Analysis
4.6. Differential Scanning Calorimetry
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, M.M.; Shi, M.J.; Zhu, W.; Yao, H.; Wang, D.-A. Polysaccharide-Based Biomaterials in Tissue Engineering: A Review. Tissue Eng. Part B Rev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Berill, D.; Al-Jwaid, A.; Caplin, J. Review Polymeric Materials Used for Immobilisation of Bacteria for the Bioremediation of Contaminants in Water. Polymers 2021, 13, 1073. [Google Scholar] [CrossRef]
- Çimen, D.; Özbek, M.; Bereli, N.; Mattiasson, B.; Denizli, A. Injectable Cryogels in Biomedicine. Gels 2021, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2019, 276, 102088. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Savina, I.N.; Mikhalovsky, S.V. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37. [Google Scholar] [CrossRef]
- Lozinsky, V. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Milakin, K.A.; Acharya, U.; Trchová, M.; Zasońska, B.A.; Stejskal, J. Polypyrrole/gelatin cryogel as a precursor for a macroporous conducting polymer. React. Funct. Polym. 2020, 157. [Google Scholar] [CrossRef]
- Abudula, T.; Colombani, T.; Alade, T.; Bencherif, S.A.; Memić, A. Injectable Lignin-co-Gelatin Cryogels with Antioxidant and Antibacterial Properties for Biomedical Applications. Biomacromolecules 2021. [Google Scholar] [CrossRef]
- Akilbekova, D.; Shaimerdenova, M.; Adilov, S.; Berillo, D. Biocompatible scaffolds based on natural polymers for regenerative medicine. Int. J. Biol. Macromol. 2018, 114, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, X.; Zhang, Z.; Liang, Y.; Yin, Z.; Chen, B.; Bai, L.; Han, Y.; Guo, B. Degradable Gelatin-Based IPN Cryogel Hemostat for Rapidly Stopping Deep Noncompressible Hemorrhage and Simultaneously Improving Wound Healing. Chem. Mater. 2020, 32, 6595–6610. [Google Scholar] [CrossRef]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-polyethylene glycol cryogels as spongy scaffolds for drug delivery. Int. J. Biol. Macromol. 2020, 166, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S. Crosslinking strategies for porous gelatin scaffolds. J. Mater. Sci. 2016, 51, 4349–4357. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Lippens, E.; Cornelissen, M.; Schacht, E. Correlation between Cryogenic Parameters and Physico-Chemical Properties of Porous Gelatin Cryogels. J. Biomater. Sci. Polym. Ed. 2009, 20, 1417–1438. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, C.; Tan, H.; Yuan, G.; Li, J.; Li, S.; Jia, Y.; Xiong, D.; Hu, X.; Niu, X. Covalently injectable chitosan/chondroitin sulfate hydrogel integrated gelatin/heparin microspheres for soft tissue engineering. Int. J. Polym. Mater. 2019, 70, 149–157. [Google Scholar] [CrossRef]
- Masatoshi, R.; Tomohiro, I.; Shingo, T.; Katsuhiro, H.; Norio, M. Water sorption and drying behavior of crosslinked dextrans. Biosci. Biotechnol. Biochem. 1999, 63, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Anvari, M.; Chung, D. Dynamic rheological and structural characterization of fish gelatin–Gum arabic coacervate gels cross-linked by tannic acid. Food Hydrocoll. 2016, 60, 516–524. [Google Scholar] [CrossRef]
- Witte, R.P.; Kao, W.J. Keratinocyte-fibroblast paracrine interaction: The effects of substrate and culture condition. Biomaterials 2005, 26, 3673–3682. [Google Scholar] [CrossRef]
- Pierce, B.F.; Pittermann, E.; Ma, N.; Gebauer, T.; Neffe, A.T.; Hölscher, M.; Jung, F.; Sablani, S.S.; Kasapis, S.; Al-Rahbi, Y.; et al. Water sorption isotherms and glass transition properties of gelatin. Dry. Technol. 2002, 20, 2081–2092. [Google Scholar] [CrossRef]
- Liao, H.-T.; Shalumon, K.T.; Chang, K.-H.; Sheua, C.; Chen, J.-P. Investigation of synergistic effects of inductive and conductive factors in gelatin-based cryogels for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1827–1841. [Google Scholar] [CrossRef]
- Foda, N.H.; El-Laithy, H.M.; Tadros, M.I. Implantable Biodegradable Sponges: Effect of Interpolymer Complex Formation of Chitosan with Gelatin on the Release Behavior of Tramadol Hydrochloride. Drug Dev. Ind. Pharm. 2007, 33, 7–17. [Google Scholar] [CrossRef]
- US Pharmacopeia Monograph on Gelatin. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m34800.html (accessed on 27 September 2021).
- US Pharmacopea Monograph Dextran. Available online: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m23770.html (accessed on 27 September 2021).
- Reichelt, S.; Becher, J.; Weisser, J.; Prager, A.; Decker, U.; Möller, S.; Berg, A.; Schnabelrauch, M. Biocompatible polysaccharide-based cryogels. Mater. Sci. Eng. C 2014, 35, 164–170. [Google Scholar] [CrossRef]
- Seker, S.; Elçin, A.E.; Elçin, Y.M. Macroporous elastic cryogels based on platelet lysate and oxidized dextran as tissue engineering scaffold: In vitro and in vivo evaluations. Mater. Sci. Eng. C 2020, 110, 110703. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Shi, H.; Zhao, G.; Zhang, Q.; Feng, C.; Xv, X. Preparation of cellulose nanocrystal/oxidized dextran/gelatin (CNC/OD/GEL) hydrogels and fabrication of a CNC/OD/GEL scaffold by 3D printing. J. Mater. Sci. 2019, 55, 2618–2635. [Google Scholar] [CrossRef]
- Andrabi, S.M.; Majumder, S.; Gupta, K.C.; Kumar, A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surfaces B Biointerfaces 2020, 195, 111263. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, G. Preparation and drug delivery of dextran-drug complex. Drug Deliv. 2019, 26, 252–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, D.N.; Alioglu, M.A.; Wu, Y.; Ozbolat, V.; Ayan, B.; Dey, M.; Kang, Y.; Ozbolat, I.T. 3D Bioprinting of Carbohydrazide-Modified Gelatin into Microparticle-Suspended Oxidized Alginate for the Fabrication of Complex-Shaped Tissue Constructs. ACS Appl. Mater. Interfaces 2020, 12, 20295–20306. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Q.; Zhan, X.; Chen, F. A multifunctional gelatin-based aerogel with superior pollutants adsorption, oil/water separation and photocatalytic properties. Chem. Eng. J. 2018, 358, 1539–1551. [Google Scholar] [CrossRef]
- López-Castejón, M.L.; Bengoechea, C.; García-Morales, M.; Martínez, I. Influence of tragacanth gum in egg white based bioplastics: Thermomechanical and water uptake properties. Carbohydr. Polym. 2016, 152, 62–69. [Google Scholar] [CrossRef]
- Yazawa, K.; Ishida, K.; Masunaga, H.; Hikima, T.; Keiji Numata, K. Influence of water content on the β-sheet formation, thermal stability, water removal, and mechanical properties of silk materials. Biomacromolecules 2016, 17, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, G.P.; Ma, Q.Y.; Gu, M.F.; Wu, X.Y.; Sheng, S.; Wang, X. Investigation on the thermo-mechanical properties and thermal stability of polylactic acid tissue engineering scaffold material. J. Therm. Anal. Calorim. 2013, 113, 1113–1121. [Google Scholar] [CrossRef]
- David, G.; Cristea, M.; Balhui, C.; Timpu, D.; Doroftei, F.; Simionescu, B.C. Effect of Cross-Linking Methods on Structure and Properties of Poly(e-caprolactone) Stabilized Hydrogels Containing Biopolymers. Biomacromolecules 2012, 13, 2263–2272. [Google Scholar] [CrossRef]
- Lewicki, P.P. Water as the determinant of food engineering properties. A review. J. Food Eng. 2004, 61, 483–495. [Google Scholar] [CrossRef]
- Carvalho, R.A.; Grosso, C.F.; Sobral, P.J.A. Effect of chemical treatment on the mechanical properties, water vapour permeability and sorption isotherms of gelatin-based films. Packag. Technol. Sci. Int. J. 2008, 21, 165–169. [Google Scholar] [CrossRef]
- Chirkova, J.; Andersons, B.; Irbe, I. Study of the structure of wood-related biopolymers by sorption methods. BioResources 2007, 4, 1044–1057. [Google Scholar]
- Gocho, H.; Shimizu, H.; Tanioka, A.; Chou, T.-J.; Nakajima, T. Effect of polymer chain end on sorption isotherm of water by chitosan. Carbohydr. Polym. 2000, 41, 87–90. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Norton, A.; Newman, G. The water vapor sorption behavior of natural fibers. J. Appl. Polym. Sci. 2009, 112, 1524–1537. [Google Scholar] [CrossRef]
- Volkova, N.; Ibrahim, V.; Hatti-Kaul, R.; Wadsö, L. Water sorption isotherms of Kraft lignin and its composites. Carbohydr. Polym. 2012, 87, 1817–1821. [Google Scholar] [CrossRef]
- Timmermann, E.O.; Chirife, J.; Iglesias, H.A. Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? J. Food Eng. 2001, 48, 19–31. [Google Scholar] [CrossRef]
- Timmermann, E.O. Multilayer sorption parameters: BET or GAB values? Colloids Surfaces A Physicochem. Eng. Asp. 2003, 220, 235–260. [Google Scholar] [CrossRef] [Green Version]
- Ludwiczak, S.; Mucha, M. Modeling of water sorption isotherms of chitosan blends. Carbohydr. Polym. 2010, 79, 34–39. [Google Scholar] [CrossRef]
- Berillo, D.A.; Volkova, N. Preparation and physicochemical characteristics of macroporous hydrogel based on gelatin and oxidized dextran. J. Mater. Sci. 2014, 49, 4855–4868. [Google Scholar] [CrossRef]
- Bancroft, W. The action of water on gelatine. J. Phys. Chem. 1911, 16, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Shull, C.A.; Shull, S.P. Adsorption of moisture in a saturated atmosphere. Am. J. Bot. 1920, 7, 318–326. [Google Scholar] [CrossRef]
- Sheppard, S.E.; Houck, R.C.; Dittmar, C. The structure of gelatin sols and gels. VI. The adsorption of water vapour and electrical conductivity. J. Physical Chem. 1940, 44, 185–207. [Google Scholar] [CrossRef]
- Texter, J.A.; Kellerman, R.; Klier, K. Water sorption in a dextran gel. Carbohydr. Res. 1975, 41, 191–210. [Google Scholar] [CrossRef]
- Taylor, N.W.; Zobel, H.F.; Hellman, N.N.; Senti, F.R. Effect of Structure and Crystallinity on Water Sorption of Dextran. J. Phys. Chem. 1959, 63, 599–603. [Google Scholar] [CrossRef]
- Hernandez, H.G.; Livigs, S.; Aguilera, J.M.; Chiralt, A. Phase transition of dairy proteins, dextrans and their mixtures as function of water interactions. Food Hydrocoll. 2011, 25, 1311–1318. [Google Scholar] [CrossRef]
- Koshy, S.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2013, 35, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Zhai, M.; Ma, F.; Li, J.; Wan, B.; Yu, N. Preparation and properties of cryogel based on poly(hydroxypropyl methacrylate). J. Biomater. Sci. Polym. Ed. 2018, 29, 1401–1425. [Google Scholar] [CrossRef]
- Livingston, H. The cross-sectional areas of molecules adsorbed on solid surfaces. J. Colloid Sci. 1949, 4, 447–458. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Adsorption of water and methanol on micro-and mesoporous wood-based activated carbons. Langmuir 2000, 16, 5435–5440. [Google Scholar] [CrossRef]
- Likos, W.J.; Lu, N. Water vapor sorption behavior of smectite-kaolinite mixtures. Clays Clay Miner. 2002, 50, 553–561. [Google Scholar] [CrossRef]
- Znamenskaya, Y.; Sotres, J.; Engblom, J.; Arnebrant, T.; Kocherbitov, V. Effect of Hydration on Structural and Thermodynamic Properties of Pig Gastric and Bovine Submaxillary Gland Mucins. J. Phys. Chem. B 2012, 116, 5047–5055. [Google Scholar] [CrossRef] [PubMed]
- Quirijns, E.J.; Van Boxtel, A.J.B.; Van Loon, W.K.P.; Van Straten, G. Sorption isotherms, GAB parameters and isosteric heat of sorption. J. Sci. Food Agric. 2005, 85, 1805–1814. [Google Scholar] [CrossRef]
- Rodriguez, N.J.; Hu, Q.; Luo, Y. Oxidized Dextran as a Macromolecular Crosslinker Stabilizes the Zein/Caseinate Nanocomplex for the Potential Oral Delivery of Curcumin. Molecules 2019, 24, 4061. [Google Scholar] [CrossRef] [Green Version]
- Nyqvist, H. Saturated salt solutions for maintaining specified relative humidities. Int. J. Pharm. Tech. Prod. Mfr. 1983, 4, 47–48. [Google Scholar] [CrossRef]



| RH, % | Gel | Dex | DDA | DDA:Gel 1:1 | DDA:Gel 1:2 | DDA:Gel 1:3 |
|---|---|---|---|---|---|---|
| 11 | 4.4 | 3.1 | 2.3 | 2.7 | 4.4 | 5.0 |
| 33 | 9.0 | 8.6 | 7.1 | 8.0 | 7.9 | 7.1 |
| 43 | 10.6 | 11.1 | 10.3 | 10.2 | 10.5 | 11.1 |
| 59 | 12.3 | 14.8 | 14.8 | 14.4 | 14.0 | 15.2 |
| 75 | 18.4 | 21.4 | 19.5 | 16.8 | 16.8 | 18.9 |
| 81 | 23.3 | 24.6 | 25.1 | 20.4 | 20.4 | 21.9 |
| 95 | 49.1 | 39.8 | 43.4 | 40.7 | 41.7 | 43.7 |
| 100 | 117.7 | 60.2 | 83.0 | 93.0 | 106.7 | 111.8 |
| Sample | Wm, g/g | S, m2/g | C | E1, kJ/mol | E1 − EEV, kJ/mol |
|---|---|---|---|---|---|
| Gelatine | 0.069 | 248 | 11.17 | 59.80 | 15.80 |
| Dextran | 0.086 | 311 | 3.83 | 33.28 | −10.72 |
| DDA | 0.092 | 334 | 2.24 | 20.00 | −24.00 |
| DDA:Gel 1:1 | 0.086 | 312 | 3.78 | 32.95 | −11.05 |
| DDA:Gel 1:2 | 0.065 | 236 | 11.38 | 60.26 | 16.26 |
| DDA:Gel 1:3 | 0.069 | 250 | 13.18 | 63.89 | 19.89 |
| Sample | WGAB, g/g | K | CGAB | Regression Coefficient |
|---|---|---|---|---|
| Gelatine | 0.0591 ± 0.0027 | 0.926 ± 0.006 | 29.8 ± 23.7 | 0.998 |
| Dextran | 0.0721 ± 0.0061 | 0.879 ± 0.011 | 11.51 ± 9.17 | 0.994 |
| DDA | 0.0614 ± 0.003 | 0.923 ± 0.003 | 9.02 ± 0.86 | 0.998 |
| DDA:Gel 1:1 | 0.0506 ± 0.003 | 0.9456 ± 0.003 | 32.2 ± 67.2 | 0.996 |
| DDA:Gel 1:2 | 0.0583 ± 0.004 | 0.9053 ± 0.012 | 33.0 ± 42.4 | 0.993 |
| DDA:Gel 1:3 | 0.0646 ± 0.005 | 0.897 ± 0.013 | 23.4 ± 24.0 | 0.993 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volkova, N.; Berillo, D. Water Uptake as a Crucial Factor on the Properties of Cryogels of Gelatine Cross-Linked by Dextran Dialdehyde. Gels 2021, 7, 159. https://doi.org/10.3390/gels7040159
Volkova N, Berillo D. Water Uptake as a Crucial Factor on the Properties of Cryogels of Gelatine Cross-Linked by Dextran Dialdehyde. Gels. 2021; 7(4):159. https://doi.org/10.3390/gels7040159
Chicago/Turabian StyleVolkova, Natalia, and Dmitriy Berillo. 2021. "Water Uptake as a Crucial Factor on the Properties of Cryogels of Gelatine Cross-Linked by Dextran Dialdehyde" Gels 7, no. 4: 159. https://doi.org/10.3390/gels7040159
APA StyleVolkova, N., & Berillo, D. (2021). Water Uptake as a Crucial Factor on the Properties of Cryogels of Gelatine Cross-Linked by Dextran Dialdehyde. Gels, 7(4), 159. https://doi.org/10.3390/gels7040159