Hydrotropic Hydrogels Prepared from Polyglycerol Dendrimers: Enhanced Solubilization and Release of Paclitaxel
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
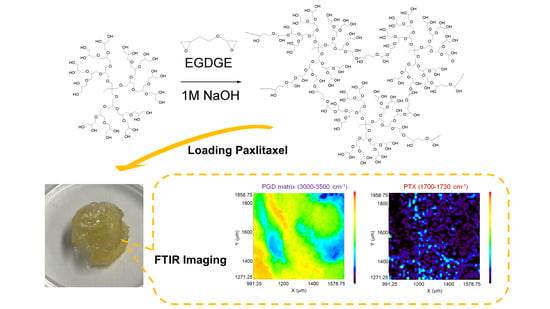
4.2. Preparation of PGD Hydrogels Crosslinked by Ethylene Glycol Diglycidyl Ether (EGDGE)
4.2.1. PGD-G3 Hydrogels Prepared in NaOH Aqueous Solution [G3-EG(NaOH)]
4.2.2. PGD-G3 Hydrogels Prepared in DMSO [G3-EG(DMSO)]
4.2.3. PGD-G4 Hydrogels Prepared in NaOH Aqueous Solution [G4-EG(NaOH)]
4.3. PTX Loading of G3-EG and G4 Hydrogels
4.4. FTIR Imaging
4.5. PTX Release from G4-EG Hydrogels
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. [Google Scholar] [CrossRef] [PubMed]
- Daniel, W.; Stiriba, S.E.; Holger, F. Hyperbranched Polyglycerols: From the Controlled Synthesis of Biocompatible Polyether Polyols to Multipurpose Applications. Acc. Chem. Res. 2010, 43, 129–141. [Google Scholar] [CrossRef]
- Bochenek, M.; Oleszko-Torbus, N.; Wałach, W.; Lipowska-Kur, D.; Dworak, A.; Utrata-Wesołek, A. Polyglycidol of Linear or Branched Architecture Immobilized on a Solid Support for Biomedical Applications. Polym. Rev. 2020, 60, 717–767. [Google Scholar] [CrossRef]
- Calderón, M.; Quadir, M.A.; Sharma, S.K.; Haag, R. Dendritic Polyglycerols for Biomedical Applications. Adv. Mater. 2010, 22, 190–218. [Google Scholar] [CrossRef] [PubMed]
- Türk, H.; Shukla, A.; Alves Rodrigues, P.C.; Rehage, H.; Haag, R. Water-Soluble Dendritic Core–Shell-Type Architectures Based on Polyglycerol for Solubilization of Hydrophobic Drugs. Chem. A Eur. J. 2007, 13, 4187–4196. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.L.; Yu, J.H.; Jun, H.; Yang, M.Y.; Yang, M.J.; Cho, J.W.; Kim, J.W.; Kim, J.S.; Nam, Y.S. Polyglycerolated Nanocarriers with Increased Ligand Multivalency for Enhanced in Vivo Therapeutic Efficacy of Paclitaxel. Biomaterials 2017, 145, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Gao, C.; Hu, X.; Xu, W. General Avenue to Multifunctional Aqueous Nanocrystals Stabilized by Hyperbranched Polyglycerol. Chem. Mater. 2011, 23, 1461–1470. [Google Scholar] [CrossRef]
- Yeh, P.-Y.J.; Kainthan, R.K.; Zou, Y.; Chiao, M.; Kizhakkedathu, J.N. Self-Assembled Monothiol-Terminated Hyperbranched Polyglycerols on a Gold Surface: A Comparative Study on the Structure, Morphology, and Protein Adsorption Characteristics with Linear Poly(Ethylene Glycol)s. Langmuir 2008, 24, 4907–4916. [Google Scholar] [CrossRef]
- Wang, X.; Gan, H.; Zhang, M.; Sun, T. Modulating Cell Behaviors on Chiral Polymer Brush Films with Different Hydrophobic Side Groups. Langmuir 2012, 28, 2791–2798. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Yang, S.; Ding, B. Growing Hyperbranched Polyglycerols on Magnetic Nanoparticles to Resist Nonspecific Adsorption of Proteins. Colloids Surf. B Biointerfaces 2008, 67, 122–126. [Google Scholar] [CrossRef]
- Wyszogrodzka, M.; Haag, R. Synthesis and Characterization of Glycerol Dendrons, Self-Assembled Monolayers on Gold: A Detailed Study of Their Protein Resistance. Biomacromolecules 2009, 10, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Sugimoto, Y.; Murakami, D.; Tanaka, M.; Ooya, T. Effect of Branching Degree of Dendritic Polyglycerols on Plasma Protein Adsorption: Relationship between Hydration States and Surface Morphology. Langmuir 2021, 37, 8534–8543. [Google Scholar] [CrossRef] [PubMed]
- Oudshoorn, M.H.M.; Rissmann, R.; Bouwstra, J.A.; Hennink, W.E. Synthesis and Characterization of Hyperbranched Polyglycerol Hydrogels. Biomaterials 2006, 27, 5471–5479. [Google Scholar] [CrossRef]
- Wu, C.; Strehmel, C.; Achazi, K.; Chiappisi, L.; Dernedde, J.; Lensen, M.C.; Gradzielski, M.; Ansorge-Schumacher, M.B.; Haag, R. Enzymatically Cross-Linked Hyperbranched Polyglycerol Hydrogels as Scaffolds for Living Cells. Biomacromolecules 2014, 15, 3881–3890. [Google Scholar] [CrossRef] [PubMed]
- Postnova, I.; Silant’ev, V.; Kim, M.H.; Song, G.Y.; Kim, I.; Ha, C.S.; Shchipunov, Y. Hyperbranched Polyglycerol Hydrogels Prepared through Biomimetic Mineralization. Colloids Surf. B Biointerfaces 2013, 103, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Steinhilber, D.; Haag, R.; Sisson, A.L. Multivalent, Biodegradable Polyglycerol Hydrogels. Int. Artif. Organs 2011, 34, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; He, G.; Zhang, L.; Lei, Q.; Guo, Y.; Fang, W. Hyperbranched Polyglycerol/Poly(Acrylic Acid) Hydrogel for the Efficient Removal of Methyl Violet from Aqueous Solutions. J. Appl. Polym. Sci. 2016, 133, 1–11. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Janzen, J.; Kizhakkedathu, J.N.; Devine, D.V.; Brooks, D.E. Hydrophobically Derivatized Hyperbranched Polyglycerol as a Human Serum Albumin Substitute. Biomaterials 2008, 29, 1693–1704. [Google Scholar] [CrossRef]
- Gao, S.; Guan, Q.; Chafeeva, I.; Brooks, D.E.; Nguan, C.Y.C.; Kizhakkedathu, J.N.; Du, C. Hyperbranched Polyglycerol as a Colloid in Cold Organ Preservation Solutions. PLoS ONE 2015, 10, e0116595. [Google Scholar] [CrossRef]
- Ooya, T.; Lee, J.; Park, K. Effects of Ethylene Glycol-Based Graft, Star-Shaped, and Dendritic Polymers on Solubilization and Controlled Release of Paclitaxel. J. Control Release 2003, 93, 121–127. [Google Scholar] [CrossRef]
- Ooya, T.; Ogawa, T.; Takeuchi, T. Temperature-Induced Recovery of a Bioactive Enzyme Using Polyglycerol Dendrimers: Correlation between Bound Water and Protein Interaction. J. Biomater. Sci. Polym. Ed. 2018, 29, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Ooya, T. Enhanced Solubilization of α-Tocopherol by Hyperbranched Polyglycerol-Modified β-Cyclodextin. J. Drug Deliv. Sci. Technol. 2016, 35, 30–33. [Google Scholar] [CrossRef]
- Park, J.H.; Huh, K.M.; Lee, S.C.; Lee, W.K.; Ooya, T.; Park, K. Nanoparticulate Drug Delivery Systems Based on Hydrotropic Polymers, Dendrimers, and Polymer Complexes. In Proceedings of the 2005 NSTI Nanotechnology Conference and Trade Show—NSTI Nanotech 2005, Anaheim, CA, USA, 8–12 May 2005; pp. 124–127. [Google Scholar]
- Ooya, T.; Huh, K.M.; Saitoh, M.; Tamiya, E.; Park, K. Self-Assembly of Cholesterol-Hydrotropic Dendrimer Conjugates into Micelle-like Structure: Preparation and Hydrotropic Solubilization of Paclitaxel. Sci. Technol. Adv. Mater. 2005, 6, 452–456. [Google Scholar] [CrossRef]
- Ooya, T.; Lee, J.; Park, K. Hydrotropic Dendrimers of Generations 4 and 5: Synthesis, Characterization, and Hydrotropic Solubilization of Paclitaxel. Bioconjug. Chem. 2004, 15, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Ooya, T.; Lee, S.C.; Huh, K.M.; Park, K. Hydrotropic Nanocarriers for Poorly Soluble Drugs. In Nanocarrier Technologies: Frontiers of Nanotherapy; Springer Nature: Cham, Switzerland, 2006; Volume 9781402050, pp. 51–73. ISBN 9781402050411. [Google Scholar]
- Appel, E.A.; Forster, R.A.; Rowland, M.J.; Scherman, O.A. The Control of Cargo Release from Physically Crosslinked Hydrogels by Crosslink Dynamics. Biomaterials 2014, 35, 9897–9903. [Google Scholar] [CrossRef]
- Pramanik, B.; Ahmed, S. Peptide-Based Low Molecular Weight Photosensitive Supramolecular Gelators. Gels 2022, 8, 533. [Google Scholar] [CrossRef]
- Pedron, S.; Pritchard, A.M.; Vincil, G.A.; Andrade, B.; Zimmerman, S.C.; Harley, B.A.C. Patterning Three-Dimensional Hydrogel Microenvironments Using Hyperbranched Polyglycerols for Independent Control of Mesh Size and Stiffness. Biomacromolecules 2017, 18, 1393–1400. [Google Scholar] [CrossRef]
- Hong, J.; Shin, Y.; Kim, S.; Lee, J.; Cha, C. Complex Tuning of Physical Properties of Hyperbranched Polyglycerol-Based Bioink for Microfabrication of Cell-Laden Hydrogels. Adv. Funct. Mater. 2019, 29, 16–19. [Google Scholar] [CrossRef]
- Kapourani, E.; Neumann, F.; Achazi, K.; Dernedde, J.; Haag, R. Droplet-Based Microfluidic Templating of Polyglycerol-Based Microgels for the Encapsulation of Cells: A Comparative Study. Macromol. Biosci. 2018, 18, 1800116. [Google Scholar] [CrossRef]
- Randriantsilefisoa, R.; Hou, Y.; Pan, Y.; Camacho, J.L.C.; Kulka, M.W.; Zhang, J.; Haag, R. Interaction of Human Mesenchymal Stem Cells with Soft Nanocomposite Hydrogels Based on Polyethylene Glycol and Dendritic Polyglycerol. Adv. Funct. Mater. 2020, 30, 1905200. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Choi, Y.; Jeena, M.T.; Ahn, E.; Choi, Y.; Kang, M.G.; Lee, C.G.; Kwon, T.H.; Rhee, H.W.; Ryu, J.H.; et al. Reduction-Triggered Self-Cross-Linked Hyperbranched Polyglycerol Nanogels for Intracellular Delivery of Drugs and Proteins. Macromol. Biosci. 2018, 18, 1700356. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.D.; Xu, C.X.; Quan, J.S.; Song, C.K.; Jin, H.; Kim, D.D.; Choi, Y.J.; Cho, M.H.; Cho, C.S. Synergistic Anti-Tumor Activity of Paclitaxel-Incorporated Conjugated Linoleic Acid-Coupled Poloxamer Thermosensitive Hydrogel in Vitro and in Vivo. Biomaterials 2009, 30, 4777–4785. [Google Scholar] [CrossRef] [PubMed]
- Singla, A.K.; Garg, A.; Aggarwal, D. Paclitaxel and Its Formulations. Int. J. Pharm. 2002, 235, 179–192. [Google Scholar] [CrossRef]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef]
- Jensen, M.; Birch Hansen, P.; Murdan, S.; Frokjaer, S.; Florence, A.T. Loading into and Electro-Stimulated Release of Peptides and Proteins from Chondroitin 4-Sulphate Hydrogels. Eur. J. Pharm. Sci. 2002, 15, 139–148. [Google Scholar] [CrossRef]
- Van Dijk-Wolthuis, W.N.E.; Franssen, O.; Talsma, H.; van Steenbergen, M.J.; Kettenes-van den Bosch, J.J.; Hennink, W.E. Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. Macromolecules 1995, 28, 6317–6322. [Google Scholar] [CrossRef]
- Salehpour, S.; Zuliani, C.J.; Dube, M.A. Synthesis of Novel Stimuli-Responsive Polyglycerol-Based Hydrogels. Eur. J. Lipid Sci. Technol. 2012, 114, 92–99. [Google Scholar] [CrossRef]
- Huh, K.M.; Lee, S.C.; Cho, Y.W.; Lee, J.; Jeong, J.H.; Park, K. Hydrotropic Polymer Micelle System for Delivery of Paclitaxel. J. Control Release 2005, 101, 59–68. [Google Scholar] [CrossRef]
- Le, Z.; Chen, Y.; Han, H.; Tian, H.; Zhao, P.; Yang, C.; He, Z.; Liu, L.; Leong, K.W.; Mao, H.-Q.; et al. Hydrogen-Bonded Tannic Acid-Based Anticancer Nanoparticle for Enhancement of Oral Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 42186–42197. [Google Scholar] [CrossRef]
- Chen, W.; Achazi, K.; Schade, B.; Haag, R. Charge-Conversional and Reduction-Sensitive Poly(Vinyl Alcohol) Nanogels for Enhanced Cell Uptake and Efficient Intracellular Doxorubicin Release. J. Control Release 2015, 205, 15–24. [Google Scholar] [CrossRef]
- Steinhilber, D.; Sisson, A.L.; Mangoldt, D.; Welker, P.; Licha, K.; Haag, R. Synthesis, Reductive Cleavage, and Cellular Interaction Studies of Biodegradable, Polyglycerol Nanogels. Adv. Funct. Mater. 2010, 20, 4133–4138. [Google Scholar] [CrossRef]
- Lee, H.; Ooya, T. Generation-Dependent Host-Guest Interactions: Solution States of Polyglycerol Dendrimers of Generations 3 and 4 Modulate the Localization of a Guest Molecule. Chem. A Eur. J. 2012, 18, 10624–10629. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Huh, K.M.; Lee, J.; Cho, Y.W.; Galinsky, R.E.; Park, K. Hydrotropic Polymeric Micelles for Enhanced Paclitaxel Solubility: In Vitro and In Vivo Characterization. Biomacromolecules 2007, 8, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.; McClean, D.; Kar, S.; Takizawa, K.; Varghese, K.; Baek, N.; Park, K.; Fishbein, M.C.; Makkar, R.; Litvack, F.; et al. Local Drug Delivery via a Coronary Stent with Programmable Release Pharmacokinetics. Circulation 2003, 107, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sample Code | Conc. of EGDGE (mmol/mL) | Conc. of PGD-G3 (wt. %) | EGDGE/OH Groups | Swelling Ratio (q) | |
|---|---|---|---|---|---|
| In Water | In Ethanol | ||||
| G3-EG(NaOH)0.15 | 0.682 | 32 | 0.15 | 5.39 | 1.16 |
| G3-EG(NaOH)0.5 | 1.261 | 18 | 0.5 | 9.12 | -- |
| G3-EG(NaOH)0.75 | 1.215 | 11 | 0.75 | 36.20 | 4.28 |
| G3-EG(DMSO)0.75 | 3.035 | 30 | 0.75 | 1.94 | -- |
| G3-EG(NaOH)1.0 | 1.621 | 11 | 1.0 | 11.39 | 3.83 |
| Sample Code | Conc. of EGDGE (mmol/mL) | Conc. of PGD-G4 (wt. %) | EGDGE/OH Groups | Swelling Ratio (q) | |
|---|---|---|---|---|---|
| In Water | In Ethanol | ||||
| G4-EG(NaOH)0.15 | 0.658 | 32 | 0.15 | 4.68 | 1.36 |
| G4-EG(NaOH)0.5 | 1.217 | 18 | 0.5 | 7.41 | 2.05 |
| G4-EG(NaOH)0.75 | 1.171 | 11 | 0.75 | 37.44 | 2.45 |
| G4-EG(NaOH)1.0 | 1.564 | 11 | 1.0 | 9.95 | 2.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooya, T.; Lee, J. Hydrotropic Hydrogels Prepared from Polyglycerol Dendrimers: Enhanced Solubilization and Release of Paclitaxel. Gels 2022, 8, 614. https://doi.org/10.3390/gels8100614
Ooya T, Lee J. Hydrotropic Hydrogels Prepared from Polyglycerol Dendrimers: Enhanced Solubilization and Release of Paclitaxel. Gels. 2022; 8(10):614. https://doi.org/10.3390/gels8100614
Chicago/Turabian StyleOoya, Tooru, and Jaehwi Lee. 2022. "Hydrotropic Hydrogels Prepared from Polyglycerol Dendrimers: Enhanced Solubilization and Release of Paclitaxel" Gels 8, no. 10: 614. https://doi.org/10.3390/gels8100614
APA StyleOoya, T., & Lee, J. (2022). Hydrotropic Hydrogels Prepared from Polyglycerol Dendrimers: Enhanced Solubilization and Release of Paclitaxel. Gels, 8(10), 614. https://doi.org/10.3390/gels8100614