Physicomechanical and Morphological Characterization of Multi-Structured Potassium-Acrylate-Based Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Infrared Spectroscopy FTIR
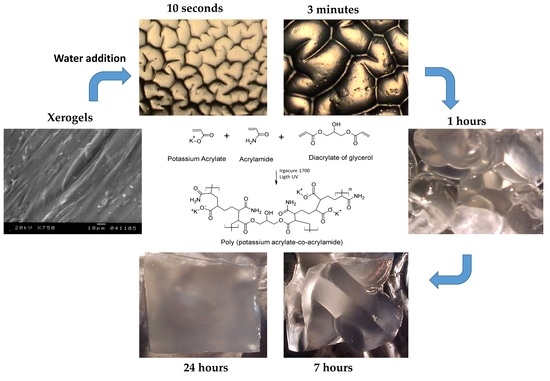
2.2. Swelling Kinetics
2.3. Surface Analysis
2.4. Mechanical Tests
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hydrogels
4.3. Swelling Kinetics
4.4. Morphological Characterization
4.5. Mechanical Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amashta, I.A.K.; Trabanca, O.K.; Trabanca, D.K. Materiales inteligentes: Hidrogeles macromoleculares. Algunas aplicaciones biomédicas. In Anales de la Real Sociedad Española de Química; Real Sociedad Española de Química: Madrid, Spain, 2005; Volume 4, pp. 35–50. [Google Scholar]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S. Superabsorbent polymers and superabsorbent polymer composites. ScienceAsia 2007, 33, 39–43. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Bautista, F.; Garcia, S.; Jimenez, R.; Lopez, L.; Orozco, E.; Prado, M.; Reyes, I. Influence of the Synthesis Thermal History on the Structure of Acrylate Based Hydrogels; ANTEC-CONFERENCE PROCEEDINGS: Boston, MA, USA, 2005; Volume 5, p. 373. [Google Scholar]
- Costa, M.C.G.; Freire, A.G.; Lourenço, D.V.; Sousa, R.R.D.; Feitosa, J.P.D.A.; Mota, J.C.A. Hydrogel composed of potassium acrylate, acrylamide, and mineral as soil conditioner under saline conditions. Sci. Agric. 2021, 79, e20200235. [Google Scholar] [CrossRef]
- Jasim, L.S.; Aljeboree, A.M. Removal of Heavy Metals by Using Chitosan/Poly (Acryl Amide-Acrylic Acid) Hydrogels: Characterization and Kinetic Study. NeuroQuantology 2021, 19, 31–38. [Google Scholar] [CrossRef]
- Zdravković, A.; Nikolić, L.; Ilić-Stojanović, S.; Nikolić, V.; Najman, S.; Mitić, Ž.; Ćirić, A.; Petrović, S. The removal of heavy metal ions from aqueous solutions by hydrogels based on N-isopropylacrylamide and acrylic acid. Polym. Bull. 2018, 75, 4797–4821. [Google Scholar] [CrossRef] [Green Version]
- Washington, R.P.; Steinbock, O. Frontal Polymerization Synthesis of Temperature-Sensitive Hydrogels. J. Am. Chem. Soc. 2001, 123, 7933–7934. [Google Scholar] [CrossRef]
- Lara-Valencia, V.A.; Dávila-Soto, H.; Moscoso-Sánchez, F.J.; Figueroa-Ochoa, E.B.; Carvajal-Ramos, F.; Fernández-Escamilla, V.V.A.; González-Álvarez, A.; Soltero-Martínez, J.F.A.; Macías-Balleza, E.R.; Enríquez, S.G. The use of polysaccharides extracted from seed of Persea americana var. Hass on the synthesis of acrylic hydrogels. Quim. Nova 2018, 41, 140–150. [Google Scholar] [CrossRef]
- Isik, B.; Kis, M. Preparation and determination of swelling behavior of poly (acrylamide-co-acrylic acid) hydrogels in water. J. Appl. Polym. Sci. 2004, 94, 1526–1531. [Google Scholar] [CrossRef]
- Thakur, A.; Wanchoo, R.K.; Singh, P. Structural parameters and swelling behavior of pH sensitive poly (acrylamide-co-acrylic acid) hydrogels. Chem. Biochem. Eng. Q. 2011, 25, 181–194. [Google Scholar]
- Cheng, W.M.; Hu, X.M.; Zhao, Y.Y.; Wu, M.Y.; Hu, Z.X.; Yu, X.T. Preparation and swelling properties of poly (acrylic acid-co-acrylamide) composite hydrogels. e-Polymers 2017, 17, 95–106. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties—A review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Argade, A.B.; Peppas, N.A. Poly (acrylic acid)-poly (vinyl alcohol) copolymers with superabsorbent properties. J. Appl. Polym. Sci. 1998, 70, 817–829. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Wongwatthanasatien, R. Superabsorbent polymer of poly [acrylamide-co-(acrylic acid)] by foamed polymerization. I. synthesis and water swelling properties. Macromol. Symp. 2004, 207, 229–240. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Tomar, R.S.; Gupta, I.; Singhal, R.; Nagpal, A.K. Synthesis of Poly (Acrylamide-co-Acrylic Acid) based Superabsorbent Hydrogels: Study of Network Parameters and Swelling Behaviour. Polym. -Plast. Technol. Eng. 2007, 46, 481–488. [Google Scholar] [CrossRef]
- Xie, J.; Liu, X.; Liang, J. Absorbency and adsorption of poly (acrylic acid-co-acrylamide) hydrogel. J. Appl. Polym. Sci. 2007, 106, 1606–1613. [Google Scholar] [CrossRef]
- Lopez-Ureta, L.C.; Orozco-Guareño, E.; Cruz-Barba, L.E.; Gonzalez-Alvarez, A.; Bautista-Rico, F. Synthesis and characterization of acrylamide/acrylic acid hydrogels crosslinked using a novel diacrylate of glycerol to produce multistructured materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2667–2679. [Google Scholar] [CrossRef]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly (acrylamide-co-acrylic acid) hydrogels. Arab. J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Amezcua, R.M.; Villanueva-Silva, R.J.; Muñoz-García, R.O.; Macias-Balleza, E.R.; Flores-Sahagun, T.H.S.; Lomelí-Ramírez, M.G.; Torres-Rendon, J.G.; Garcia-Enriquez, S. Preparation of Agave tequilana Weber Nanocrystalline Cellulose and its Use as Reinforcement for Acrylic Hydrogels. BioResources 2021, 16, 2731–2746. [Google Scholar] [CrossRef]
- Mironi-Harpaz, I.; Wang, D.Y.; Venkatraman, S.; Seliktar, D. Photopolymerization of cell-encapsulating hydrogels: Crosslinking efficiency versus cytotoxicity. Acta Biomater. 2012, 8, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, A.K.; Bowman, C.N. Impact of Oxygen on Photopolymerization Kinetics and Polymer Structure. Macromolecules 2006, 39, 2501–2506. [Google Scholar] [CrossRef]
- O’Brien, A.K.; Bowman, C.N. Modeling the Effect of Oxygen on Photopolymerization Kinetics. Macromol. Theory Simul. 2006, 15, 176–182. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Allonas, X.; Burget, D. Photopolymerization reactions under visible lights: Principle, mechanisms and examples of applications. Prog. Org. Coat. 2003, 47, 16–36. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Photopolymerization of multifunctional monomers in condensed phase. J. Appl. Polym. Sci. 1987, 34, 1603–1618. [Google Scholar] [CrossRef]
- Rodgers, Z.L.; Hughes, R.M.; Doherty, L.M.; Shell, J.R.; Molesky, B.P.; Brugh, A.M.; Forbes, M.D.E.; Moran, A.M.; Lawrence, D.S. B12-Mediated, Long Wavelength Photopolymerization of Hydrogels. J. Am. Chem. Soc. 2015, 137, 3372–3378. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Zhang, J.; Wang, Z.; Chen, S. Development of robust biocompatible silicone with high resistance to protein adsorption and bacterial adhesion. Acta Biomater. 2011, 7, 2053–2059. [Google Scholar] [CrossRef]
- Weiqing, R.; Jinliang, Q.; Yuli, H.; Aijie, N. Superabsorbent resin of acrylic acid/ammonium acrylate copolymers synthesized by ultraviolet photopolymerization. J. Appl. Polym. Sci. 2004, 95, 546–555. [Google Scholar] [CrossRef]
- Wen, Y.; Zhu, X.; Gauthier, D.E.; An, X.; Cheng, D.; Ni, Y.; Yin, L. Development of poly (acrylic acid)/nanofibrillated cellulose superabsorbent composites by ultraviolet light induced polymerization. Cellulose 2015, 22, 2499–2506. [Google Scholar] [CrossRef]
- Martínez-Salcedo, S.L.; Torres-Rendón, J.G.; García-Enriquez, S.; Anzaldo-Hernández, J.; Silva-Guzmán, J.A.; de Muniz, G.I.B.; Lomelí-Ramírez, M.G. Physicomechanical Characterization of Poly (acrylic acid-co-acrylamide) Hydrogels Reinforced with TEMPO-oxidized Blue Agave Cellulose Nanofibers. Fibers Polym. 2022, 23, 1161–1170. [Google Scholar] [CrossRef]
- Ruan, W.; Qiao, J.; Huang, Y.; Niu, A. Synthesis of superabsorbent resin by ultraviolet photopolymerization. J. Appl. Polym. Sci. 2004, 92, 1618–1624. [Google Scholar] [CrossRef]
- Trujillo, V.; Kim, J.; Hayward, R.C. Creasing instability of surface-attached hydrogels. Soft Matter 2008, 4, 564–569. [Google Scholar] [CrossRef]
- Tanaka, T.; Sun, S.T.; Hirokawa, Y.; Katayama, S.; Kucera, J.; Hirose, Y.; Amiya, T. Mechanical instability of gels at the phase transition. Nature 1987, 325, 796–798. [Google Scholar] [CrossRef]
- Tanaka, H.; Tomita, H.; Takasu, A.; Hayashi, T.; Nishi, T. Morphological and kinetic evolution of surface patterns in gels during the swelling process: Evidence of dynamic pattern ordering. Phys. Rev. Lett. 1992, 68, 2794–2797. [Google Scholar] [CrossRef]
- Li, C.; Hu, Z.; Li, Y. Temperature and time dependencies of surface patterns in constrained ionic N-isopropylacrylamide gels. J. Chem. Phys. 1994, 100, 4645–4652. [Google Scholar] [CrossRef]
- Guvendiren, M.; Yang, S.; Burdick, J.A. Swelling-induced surface patterns in hydrogels with gradient crosslinking density. Adv. Funct. Mater. 2009, 19, 3038–3045. [Google Scholar] [CrossRef]
- Guvendiren, M.; Burdick, J.A.; Yang, S. Solvent induced transition from wrinkles to creases in thin film gels with depth-wise crosslinking gradients. Soft Matter 2010, 6, 5795–5801. [Google Scholar] [CrossRef]
- Chuang, Y.F.; Wei, M.K.; Yang, F.; Lee, S. Water-driven surface wrinkling of poly (2-hydroxyethyl methacrylate) after ultraviolet irradiation. J. Polym. Res. 2021, 28, 1–11. [Google Scholar] [CrossRef]
- Saha, K.; Kim, J.; Irwin, E.; Yoon, J.; Momin, F.; Trujillo, V.; Schaffeer, D.V.; Healy, K.E.; Hayward, R.C. Surface creasing instability of soft polyacrylamide cell culture substrates. Biophys. J. 2010, 99, L94–L96. [Google Scholar] [CrossRef] [Green Version]
- Stafford, C.M.; Harrison, C.; Beers, K.L.; Karim, A.; Amis, E.J.; VanLandingham, M.R.; Kim, H.C.; Volksen, W.; Miller, R.D.; Simonyi, E.E. A buckling-based metrology for measuring the elastic moduli of polymeric thin films. Nat. Mater. 2004, 3, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Sidorenko, A.; Krupenkin, T.; Taylor, A.; Fratzl, P.; Aizenberg, J. Reversible switching of hydrogel-actuated nanostructures into complex micropatterns. Science 2007, 315, 487–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, D.P.; Ursiny, M.; Crosby, A.J. Crumpled surface structures. Soft Matter 2008, 4, 82–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Holmes, D.P. Swelling-induced deformations: A materials-defined transition from macroscale to microscale deformations. Soft Matter 2013, 9, 5524–5528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phadnis, A.; Manning, K.C.; Sanders, I.; Burgin, T.P.; Rykaczewski, K. Droplet-train induced spatiotemporal swelling regimes in elastomers. Soft Matter 2018, 14, 5869–5877. [Google Scholar] [CrossRef]
- Stockmayer, W.H. Theory of molecular size distribution and gel formation in branched polymers II. General cross linking. J. Chem. Phys. 1944, 12, 125–131. [Google Scholar] [CrossRef]
- Magalhães, A.S.G.; Almeida Neto, M.P.; Bezerra, M.N.; Ricardo, N.M.; Feitosa, J. Application of FTIR in the determination of acrylate content in poly (sodium acrylate-co-acrylamide) superabsorbent hydrogels. Química Nova 2012, 35, 1464–1467. [Google Scholar] [CrossRef]
- Leitão, R.C.; Moura, C.P.D.; da Silva, L.R.; Ricardo, N.M.; Feitosa, J.; Muniz, E.C.; Fajardo, R.A.; Rodrigues, F.H. Novel superabsorbent hydrogel composite based on poly (acrylamide-co-acrylate)/nontronite: Characterization and swelling performance. Química Nova 2015, 38, 370–377. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. Part B 1992, 31, 1–9. [Google Scholar] [CrossRef]
- Katime, I.; Velada, J.L.; Novoa, R.; Díaz de Apodaca, E.; Puig, J.; Mendizabal, E. Swelling Kinetics of Poly (acrylamide)/Poly (mono-n-alkyl itaconates) Hydrogels. Polym. Int. 1996, 40, 281–286. [Google Scholar] [CrossRef]
- ASTM International. D638-14 Standard Test Method for Tensile Properties of Plastics; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]









| Concentration of DAG (wt %) | Polymer Gel Fraction, GF (%) | Maximum Swelling | K × 108 | Characteristic Length, λ × 106 m | Tensile Strength (Pa) | Young’s Modulus (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| 0.5 | 91.2 ± 0.8 | 216.2 ± 7.5 | 4.28 ± 0.15 | 1.46 ± 0.038 | 1979 ± 19 | 2.64 ± 0.09 | 1647 ± 52.4 |
| 1.0 | 92.5 ± 1.1 | 155.1 ± 5.9 | 4.72 ± 0.18 | 1.41± 0.036 | 2087 ± 17 | 3.66 ± 0.10 | 1036 ± 43.1 |
| 2.0 | 92.4 ± 0.9 | 136.8 ± 4.0 | 5.62 ± 0.16 | 1.34 ± 0.039 | 2121 ± 32 | 3.73 ± 0.18 | 871 ± 36.0 |
| 3.0 | 93.6 ± 1.4 | 120.2 ± 4.3 | 6.03 ± 0.21 | 1.18 ± 0.025 | 2206 ± 16 | 6.48 ± 0.19 | 660 ± 34.3 |
| 4.0 | 92.1 ± 0.9 | 99.7 ± 3.9 | 5.54 ± 0.22 | 1.08 ± 0.031 | 2262 ± 34 | 7.80 ± 0.22 | 517 ± 32.0 |
| 5.0 | 93.4 ± 1.2 | 93.7 ± 3.4 | 5.93 ± 0.21 | 1.04 ± 0.016 | 2261 ± 28 | 8.84 ± 0.43 | 391 ± 15.2 |
| 6.0 | 93.1 ± 1.3 | 93.0 ± 3.5 | 6.86 ± 0.26 | 0.99 ± 0.007 | 2343 ± 24 | 13.43 ± 0.38 | 349 ± 23.7 |
| 7.0 | 92.9 ± 1.4 | 79.2 ± 2.3 | 8.08 ± 0.23 | 0.97 ± 0.008 | 2427 ± 20 | 14.38 ± 0.86 | 279 ± 21.0 |
| 8.0 | 91.6 ± 0.9 | 75.0 ± 2.7 | 7.38 ± 0.26 | 0.95 ± 0.005 | 2435 ± 18 | 15.25 ± 0.44 | 251 ± 15.5 |
| 9.0 | 92.0 ± 0.9 | 70.7 ± 2.8 | 8.34 ± 0.33 | 0.92 ± 0.008 | 2482 ± 26 | 22.34 ± 1.45 | 210 ± 19.2 |
| 10.0 | 92.7 ± 1.4 | 69.4 ± 3.3 | 10.21 ± 0.49 | 0.91 ± 0.006 | 2515 ± 33 | 28.47 ± 0.81 | 128 ± 12.5 |
| Substance | Amount (g) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acrylamide | 49.0 | ||||||||||
| Acrylic acid | 51.0 | ||||||||||
| DAG | 0.5 | 1.0 | 2.0 | 3.0 | 4.0 | 5.0 | 6.0 | 7.0 | 8.0 | 9.0 | 10.0 |
| Irgacure 1700 | 0.03 | ||||||||||
| Water | 100.0 | ||||||||||
| KOH | neutralizer and generating potassium acrylate | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradilla-Orozco, J.L.; Hernández-Jiménez, J.Á.; Robles-Vásquez, O.; Cortes-Ortega, J.A.; Renteria-Urquiza, M.; Lomelí-Ramírez, M.G.; Rendón, J.G.T.; Jiménez-Amezcua, R.M.; García-Enriquez, S. Physicomechanical and Morphological Characterization of Multi-Structured Potassium-Acrylate-Based Hydrogels. Gels 2022, 8, 627. https://doi.org/10.3390/gels8100627
Gradilla-Orozco JL, Hernández-Jiménez JÁ, Robles-Vásquez O, Cortes-Ortega JA, Renteria-Urquiza M, Lomelí-Ramírez MG, Rendón JGT, Jiménez-Amezcua RM, García-Enriquez S. Physicomechanical and Morphological Characterization of Multi-Structured Potassium-Acrylate-Based Hydrogels. Gels. 2022; 8(10):627. https://doi.org/10.3390/gels8100627
Chicago/Turabian StyleGradilla-Orozco, José Luis, José Ángel Hernández-Jiménez, Oscar Robles-Vásquez, Jorge Alberto Cortes-Ortega, Maite Renteria-Urquiza, María Guadalupe Lomelí-Ramírez, José Guillermo Torres Rendón, Rosa María Jiménez-Amezcua, and Salvador García-Enriquez. 2022. "Physicomechanical and Morphological Characterization of Multi-Structured Potassium-Acrylate-Based Hydrogels" Gels 8, no. 10: 627. https://doi.org/10.3390/gels8100627
APA StyleGradilla-Orozco, J. L., Hernández-Jiménez, J. Á., Robles-Vásquez, O., Cortes-Ortega, J. A., Renteria-Urquiza, M., Lomelí-Ramírez, M. G., Rendón, J. G. T., Jiménez-Amezcua, R. M., & García-Enriquez, S. (2022). Physicomechanical and Morphological Characterization of Multi-Structured Potassium-Acrylate-Based Hydrogels. Gels, 8(10), 627. https://doi.org/10.3390/gels8100627