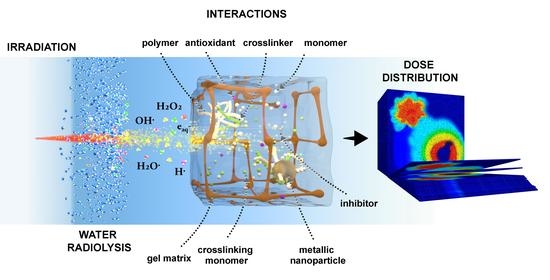
Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review
Abstract
:1. Introduction
2. Brief History of Gel Dosimetry
3. Readout Techniques
4. Radio Physical Processes
5. Radiochemical Modeling of Polymer Gel Dosimetry
6. Operational Properties of Gel Dosimetry
6.1. Sensitivity, Dynamic Range, and Minimum Detectable Dose
6.2. Stability and Reproducibility
6.3. Energy and Dose Rate Dependency
6.4. Accuracy and Precision
6.5. Water Equivalence
7. Chemical and Physical Interactions Present in Gel Dosimetry
7.1. Gel Matrix
7.2. Cosolvents
7.3. Antioxidants, Oxygen Scavengers, and Inhibitors
7.4. Nanoparticles
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, L.J.; Lilley, J. Radiotherapy: Technical Aspects. Medicine 2020, 48, 79–83. [Google Scholar] [CrossRef]
- De Deene, Y. Radiation Dosimetry by Use of Radiosensitive Hydrogels and Polymers: Mechanisms, State-of-the-Art and Perspective from 3D to 4D. Gels 2022, 8, 599. [Google Scholar] [CrossRef]
- Romero, M.; Macchione, M.A.; Mattea, F.; Strumia, M. The Role of Polymers in Analytical Medical Applications. A Review. Microchem. J. 2020, 159, 105366. [Google Scholar] [CrossRef]
- Fiorino, C.; Guckemberger, M.; Schwarz, M.; van der Heide, U.A.; Heijmen, B. Technology-Driven Research for Radiotherapy Innovation. Mol. Oncol. 2020, 14, 1500–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.H.; Kim, J.H.; Kim, J.I.; Park, J.M. Comparison of Treatment Plan Quality among MRI-Based IMRT with a Linac, MRI-Based IMRT with Tri-Co-60 Sources, and VMAT for Spine SABR. PLoS ONE 2019, 14, e0220039. [Google Scholar] [CrossRef]
- Schreiner, L.J. True 3D Chemical Dosimetry (Gels, Plastics): Development and Clinical Role. J. Phys. Conf. Ser. 2015, 573, 012003. [Google Scholar] [CrossRef]
- Azadbakht, B.; Hadad, K.; Zahmatkesh, M.H. Response Verification of Dose Rate and Time Dependence of PAGAT Polymer Gel Dosimeters by Photon Beams Using Magnetic Resonance Imaging. J. Phys. Conf. Ser. 2009, 164, 012036. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- Doran, S.; Gorjiara, T.; Kacperek, A.; Adamovics, J.; Kuncic, Z.; Baldock, C. Issues Involved in the Quantitative 3D Imaging of Proton Doses Using Optical CT and Chemical Dosimeters. Phys. Med. Biol. 2015, 60, 709–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambarini, G.; Bettega, D.; Camoni, G.; Felisi, M.; Gebbia, A.; Massari, E.; Regazzoni, V.; Veronese, I.; Giove, D.; Mirandola, A.; et al. Correction Method of Measured Images of Absorbed Dose for Quenching Effects Due to Relatively High LET. Radiat. Phys. Chem. 2017, 140, 15–19. [Google Scholar] [CrossRef]
- Gorjiara, T.; Kuncic, Z.; Baldock, C. SU-E-T-149: 3D Proton Gel Dosimetry. Med. Phys. 2011, 38, 3520. [Google Scholar] [CrossRef]
- Marrale, M.; D’errico, F. Hydrogels for Three-Dimensional Ionizing-Radiation Dosimetry. Gels 2021, 7, 74. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, L.; Chen, H.; Hu, L. Recent Advances in Hydrogel-Based Sensors Responding to Ionizing Radiation. Gels 2022, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Alyani Nezhad, Z.; Geraily, G. A Review Study on Application of Gel Dosimeters in Low Energy Radiation Dosimetry. Appl. Radiat. Isot. 2022, 179, 110015. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Geraily, G.; Abtahi, S.M.M. A Systematic Review of Clinical Applications of Polymer Gel Dosimeters in Radiotherapy. Appl. Radiat. Isot. 2019, 143, 47–59. [Google Scholar] [CrossRef]
- Fricke, H.; Morse, S. The Chemical Action of Roentgen Rays on Dilute Ferrous Sulfate Solutions as a Measure of Radiation Dose. J. Roentgenol. Radium Ther. Nucl. Med. 1927, 18, 430–432. [Google Scholar]
- Gore, J.C.; Kang, Y.S. Measurement of Radiation Dose Distributions by Nuclear Magnetic Resonance (NMR) Imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, L.J. Review of Fricke Gel Dosimeters. J. Phys. Conf. Ser. 2004, 3, 9–21. [Google Scholar] [CrossRef]
- Gupta, B.L.; Gomathy, K.R. Consistency of Ferrous Sulphate-Benzoic Acid-Xylenol Orange Dosimeter. Int. J. Appl. Radiat. Isot. 1974, 25, 509–513. [Google Scholar] [CrossRef]
- Bero, M.A.; Gilboy, W.B.; Glover, P.M.; El-Masri, H.M. Tissue-Equivalent Gel for Non-Invasive Spatial Radiation Dose Measurements. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 2000, 166, 820–825. [Google Scholar] [CrossRef]
- Del Lama, L.S.; de Góes, E.G.; Petchevist, P.C.D.; Moretto, E.L.; Borges, J.C.; Covas, D.T.; de Almeida, A. Prevention of Transfusion-Associated Graft-versus-Host Disease by Irradiation: Technical Aspect of a New Ferrous Sulphate Dosimetric System. PLoS ONE 2013, 8, e65334. [Google Scholar] [CrossRef]
- Chu, K.C.; Jordan, K.J.; Battista, J.J.; Van Dyk, J.; Rutt, B.K. Polyvinyl Alcohol-Fricke Hydrogel and Cryogel: Two New Gel Dosimetry Systems with Low Fe3+ Diffusion. Phys. Med. Biol. 2000, 45, 955–969. [Google Scholar] [CrossRef]
- Marini, A.; Lazzeri, L.; Cascone, M.G.; Ciolini, R.; Tana, L.; d’Errico, F. Fricke Gel Dosimeters with Low-Diffusion and High-Sensitivity Based on a Chemically Cross-Linked PVA Matrix. Radiat. Meas. 2017, 106, 618–621. [Google Scholar] [CrossRef]
- Marrale, M.; Collura, G.; Gallo, S.; Nici, S.; Tranchina, L.; Abbate, B.F.; Marineo, S.; Caracappa, S.; d’Errico, F. Analysis of Spatial Diffusion of Ferric Ions in PVA-GTA Gel Dosimeters through Magnetic Resonance Imaging. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2017, 396, 50–55. [Google Scholar] [CrossRef]
- Gallo, S.; Lizio, D.; Monti, A.F.; Veronese, I.; Brambilla, M.G.; Lenardi, C.; Torresin, A.; Gambarini, G. Temperature Behavior of Radiochromic Poly(Vinyl-Alcohol)-Glutaraldehyde Fricke Gel Dosimeters in Practice. J. Phys. D Appl. Phys. 2020, 53, 365003. [Google Scholar] [CrossRef]
- Gallo, S.; Artuso, E.; Brambilla, M.G.; Gambarini, G.; Lenardi, C.; Monti, A.F.; Torresin, A.; Pignoli, E.; Veronese, I. Characterization of Radiochromic Poly(Vinyl-Alcohol)-Glutaraldehyde Fricke Gels for Dosimetry in External X-ray Radiation Therapy. J. Phys. D Appl. Phys. 2019, 52, 225601. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Eyadeh, M.M.; Hailat, T.F.; Madas, B.G.; Aldweri, F.M.; Almomani, A.M.; Awad, S.I. Improvement on the Performance of Chemically Cross-Linked Fricke Methylthymol-Blue Radiochromic Gel Dosimeter by Addition of Dimethyl Sulfoxide. Radiat. Meas. 2021, 141, 106540. [Google Scholar] [CrossRef]
- Lazzeri, L.; Marini, A.; Cascone, M.G.; D’Errico, F. Dosimetric and Chemical Characteristics of Fricke Gels Based on PVA Matrices Cross-Linked with Glutaraldehyde. Phys. Med. Biol. 2019, 64, 085015. [Google Scholar] [CrossRef]
- Alexander, P.; Charlesby, A.; Ross, M. The Degradation of Solid Polymethylmethacrylate by Ionizing Radiation. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1954, 223, 392–404. [Google Scholar] [CrossRef]
- Boni, A.L. A Polyacrylamide Gamma Dosimeter. Radiat. Res. 1961, 14, 374. [Google Scholar] [CrossRef]
- Hoecker, F.E.; Watkins, I.W. Radiation Polymerization Dosimetry. Int. J. Appl. Radiat. Isot. 1958, 3, 31–35. [Google Scholar] [CrossRef]
- Kennan, R.P.; Maryanski, M.J.; Zhong, J.; Gore, J.C. Hydrodynamic Effects and Cross Relaxation in Cross Linked Polymer Gels. In Proceedings of the International Society for Magnetic Resonance in Medicine, Berlin, Germany, 8–14 August 1992; John Wiley & Sons, Inc.: New York, NY, USA, 1992; p. 1316. [Google Scholar]
- Maryanski, M.J.; Schulz, R.J.; Ibbott, G.S.; Gatenby, J.C.; Xie, J.; Horton, D.; Gore, J.C. Magnetic Resonance Imaging of Radiation Dose Distributions Using a Polymer-Gel Dosimeter. Phys. Med. Biol. 1994, 39, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.M.; Keil, D.C.; Does, M.D.; Gore, J.C. Polymer Gels for Magnetic Resonance Imaging of Radiation Dose Distributions at Normal Room Atmosphere. Phys. Med. Biol. 2001, 46, 3105–3113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Deene, Y.; Hurley, C.; Venning, A.; Vergote, K.; Mather, M.; Healy, B.J.; Baldock, C. A Basic Study of Some Normoxic Polymer Gel Dosimeters. Phys. Med. Biol. 2002, 47, 3441–3463. [Google Scholar] [CrossRef]
- Yao, C.H.; Chang, T.H.; Su, C.T.; Lai, Y.C.; Hsu, S.M.; Chen, C.H.; Chang, Y.J. A Study of Dose Verification and Comparison for Complex Irradiation Field with High Dose Rate Radiation by Using a 3D N-Isopropylacrylamide Gel Dosimeter. J. Radioanal. Nucl. Chem. 2019, 322, 1287–1297. [Google Scholar] [CrossRef]
- Elter, A.; Dorsch, S.; Mann, P.; Runz, A.; Johnen, W.; Spindeldreier, C.K.; Klüter, S.; Karger, C.P. End-to-End Test of an Online Adaptive Treatment Procedure in MR-Guided Radiotherapy Using a Phantom with Anthropomorphic Structures. Phys. Med. Biol. 2019, 64, 225003. [Google Scholar] [CrossRef] [Green Version]
- Hillbrand, M.; Landry, G.; Ebert, S.; Dedes, G.; Pappas, E.; Kalaitzakis, G.; Kurz, C.; Würl, M.; Englbrecht, F.; Dietrich, O.; et al. Gel Dosimetry for Three Dimensional Proton Range Measurements in Anthropomorphic Geometries. Z. Med. Phys. 2019, 29, 162–172. [Google Scholar] [CrossRef]
- Watanabe, Y.; Mizukami, S.; Eguchi, K.; Maeyama, T.; Hayashi, S.I.; Muraishi, H.; Terazaki, T.; Gomi, T. Dose Distribution Verification in High-Dose-Rate Brachytherapy Using a Highly Sensitive Normoxic N-Vinylpyrrolidone Polymer Gel Dosimeter. Phys. Med. 2019, 57, 72–79. [Google Scholar] [CrossRef]
- Abtahi, S.M.M.; Kargar Shaker Langaroodi, R.; Akbari, M.E. Dose Distribution Verification in Intraoperative Radiation Therapy Using an N-Isopropyl Acrylamide-Based Polymer Gel Dosimeter. J. Radioanal. Nucl. Chem. 2020, 324, 481–488. [Google Scholar] [CrossRef]
- Chou, Y.H.; Lu, Y.C.; Peng, S.L.; Lee, S.C.; Hsieh, L.L.; Shih, C.T. Evaluation of the Dose Distribution of Tomotherapy Using Polymer Gel Dosimeters and Optical Computed Tomography with Ring Artifact Correction. Radiat. Phys. Chem. 2020, 168, 108572. [Google Scholar] [CrossRef]
- Kozicki, M.; Berg, A.; Maras, P.; Jaszczak, M.; Dudek, M. Clinical Radiotherapy Application of N-Vinylpyrrolidone-Containing 3D Polymer Gel Dosimeters with Remote External MR-Reading. Phys. Med. 2020, 69, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Umeh, C.; Oldham, M.; Floyd, S.; Giles, W.; Adamson, J. Comprehensive Radiation and Imaging Isocenter Verification Using NIPAM KV-CBCT Dosimetry. Med. Phys. 2020, 47, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Mann, P.; Witte, M.; Mercea, P.; Nill, S.; Lang, C.; Karger, C.P. Feasibility of Markerless Fluoroscopic Real-Time Tumor Detection for Adaptive Radiotherapy: Development and End-To-End Testing. Phys. Med. Biol. 2020, 65, 115002. [Google Scholar] [CrossRef] [PubMed]
- Schwahofer, A.; Mann, P.; Spindeldreier, C.K.; Karger, C.P. On the Feasibility of Absolute 3D Dosimetry Using LiF Thermoluminescence Detectors and Polymer Gels on a 0.35T MR-LINAC. Phys. Med. Biol. 2020, 65, 215002. [Google Scholar] [CrossRef]
- Elter, A.; Rippke, C.; Johnen, W.; Mann, P.; Hellwich, E.; Schwahofer, A.; Dorsch, S.; Buchele, C.; Klüter, S.; Karger, C.P. End-to-End Test for Fractionated Online Adaptive MR-Guided Radiotherapy Using a Deformable Anthropomorphic Pelvis Phantom. Phys. Med. Biol. 2021, 66, 245021. [Google Scholar] [CrossRef]
- Alyani Nezhad, Z.; Geraily, G.; Zohari, S. Investigation of Isotropic Radiation of Low Energy X-ray Intra-Operative Radiotherapy by MAGAT Gel Dosimeter. Radiat. Phys. Chem. 2021, 188, 109648. [Google Scholar] [CrossRef]
- Azadeh, P.; Amiri, S.; Mostaar, A.; Yaghobi Joybari, A.; Paydar, R. Evaluation of MAGIC-f Polymer Gel Dosimeter for Dose Profile Measurement in Small Fields and Stereotactic Irradiation. Radiat. Phys. Chem. 2022, 194, 109991. [Google Scholar] [CrossRef]
- Fuse, H.; Oyama, S.; Fujisaki, T.; Yasue, K.; Hanada, K.; Tomita, F.; Abe, S. Mouthpiece Polymer-Gel Dosimeter for in Vivo Oral Dosimetry during Head and Neck Radiotherapy. Appl. Radiat. Isot. 2022, 186, 110301. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.; Shin, W.G.; Son, J.; Choi, C.H.; Park, J.M.; Hwang, U.J.; Kim, J.; Jung, S. 3D Star Shot Analysis Using MAGAT Gel Dosimeter for Integrated Imaging and Radiation Isocenter Verification of MR-Linac System. J. Appl. Clin. Med. Phys. 2022, 23, e13615. [Google Scholar] [CrossRef]
- Kudrevicius, L.; Jaselske, E.; Adliene, D.; Rudzianskas, V.; Radziunas, A.; Tamasauskas, A. Application of 3D Gel Dosimetry as a Quality Assurance Tool in Functional Leksell Gamma Knife Radiosurgery. Gels 2022, 8, 69. [Google Scholar] [CrossRef]
- Watanabe, Y.; Maeyama, T.; Mizukami, S.; Tachibana, H.; Terazaki, T.; Takei, H.; Muraishi, H.; Gomi, T.; Hayashi, S. Verification of Dose Distribution in High Dose-Rate Brachytherapy for Cervical Cancer Using a Normoxic N -Vinylpyrrolidone Polymer Gel Dosimeter. J. Radiat. Res. 2022, rrac053. [Google Scholar] [CrossRef]
- Shirato, H.; Shimizu, S.; Shimizu, T.; Nishioka, T.; Miyasaka, K. Real-Time Tumour-Tracking Radiotherapy. Lancet 1999, 353, 1331–1332. [Google Scholar] [CrossRef]
- Keall, P.J.; Nguyen, D.T.; O’Brien, R.; Zhang, P.; Happersett, L.; Bertholet, J.; Poulsen, P.R. Review of Real-Time 3-Dimensional Image Guided Radiation Therapy on Standard-Equipped Cancer Radiation Therapy Systems: Are We at the Tipping Point for the Era of Real-Time Radiation Therapy? Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Hilts, M.; Audet, C.; Duzenli, C.; Jirasek, A. Polymer Gel Dosimetry Using X-ray Computed Tomography: A Feasibility Study. Phys. Med. Biol. 2000, 45, 2559–2571. [Google Scholar] [CrossRef] [PubMed]
- Jirasek, A.; Hilts, M.; McAuley, K.B. Polymer Gel Dosimeters with Enhanced Sensitivity for Use in X-ray CT Polymer Gel Dosimetry. Phys. Med. Biol. 2010, 55, 5269–5281. [Google Scholar] [CrossRef]
- Chain, J.N.M.; Jirasek, A.; Schreiner, L.J.; McAuley, K.B. Cosolvent-Free Polymer Gel Dosimeters with Improved Dose Sensitivity and Resolution for X-ray CT Dose Response. Phys. Med. Biol. 2011, 56, 2091–2102. [Google Scholar] [CrossRef]
- Javaheri, N.; Yarahmadi, M.; Refaei, A.; Aghamohammadi, A. Improvement of Sensitivity of X-ray CT Reading Method for Polymer Gel in Radiation Therapy. Rep. Pract. Oncol. Radiother. 2020, 25, 100–103. [Google Scholar] [CrossRef]
- Jirasek, A.; Marshall, J.; Mantella, N.; Diaco, N.; Maynard, E.; Teke, T.; Hilts, M. Linac-Integrated KV-Cone Beam CT Polymer Gel Dosimetry. Phys. Med. Biol. 2020, 65, 225030. [Google Scholar] [CrossRef]
- Vandecasteele, J.; De Deene, Y. On the Validity of 3D Polymer Gel Dosimetry: III. MRI-Related Error Sources. Phys. Med. Biol. 2013, 58, 63–85. [Google Scholar] [CrossRef]
- De Deene, Y. Review of Quantitative MRI Principles for Gel Dosimetry. J. Phys. Conf. Ser. 2009, 164, 012033. [Google Scholar] [CrossRef]
- Maraghechi, B.; Gach, H.M.; Setianegara, J.; Yang, D.; Li, H.H. Dose Uncertainty and Resolution of Polymer Gel Dosimetry Using an MRI Guided Radiation Therapy System’s Onboard 0.35 T Scanner. Phys. Med. 2020, 73, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Jirasek, A. Considerations for X-ray CT Polymer Gel Dosimetry. J. Phys. Conf. Ser. 2013, 444, 012005. [Google Scholar] [CrossRef] [Green Version]
- Johnston, H.; Hilts, M.; Carrick, J.; Jirasek, A. An X-ray CT Polymer Gel Dosimetry Prototype: II. Gel Characterization and Clinical Application. Phys. Med. Biol. 2012, 57, 3155–3175. [Google Scholar] [CrossRef]
- Oldham, M.; Kim, L. Optical-CT Gel-Dosimetry II: Optical Artifacts and Geometrical Distortion. Med. Phys. 2004, 31, 1093–1104. [Google Scholar] [CrossRef] [Green Version]
- Jordan, K. Advances in Optical CT Scanning for Gel Dosimetry. J. Phys. Conf. Ser. 2004, 3, 115–121. [Google Scholar] [CrossRef]
- Senthilkumar, D.; James Jabaseelan, S.E. X-ray and Optical CT Analysis on Normoxic PAGAT Gel Dosimeter: An Initial Analysis & Experience; Lap Lambert Academic Publishing: Chisinau, Moldavia, 2014; ISBN 3659536261. [Google Scholar]
- Rintoul, L.; Lepage, M.; Baldock, C. Radiation Dose Distribution in Polymer Gels by Raman Spectroscopy. Appl. Spectrosc. 2003, 57, 51–57. [Google Scholar] [CrossRef]
- Jirasek, A. Alternative Imaging Modalities for Polymer Gel Dosimetry. J. Phys. Conf. Ser. 2010, 250, 338–348. [Google Scholar] [CrossRef]
- Chacón, D.; Vedelago, J.; Strumia, M.C.; Valente, M.; Mattea, F. Raman Spectroscopy as a Tool to Evaluate Oxygen Effects on the Response of Polymer Gel Dosimetry. Appl. Radiat. Isot. 2019, 150, 43–52. [Google Scholar] [CrossRef]
- Attix, H.F. Introduction to Radiological Physics and Radiation Dosimetry; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Leroy, C.; Rancoita, P.G. Principles of Radiation Interaction in Matter and Detection, Third Edition, 3rd ed.; World Scientific Publishing Company: Singapore, 2011; ISBN 9789814360524. [Google Scholar]
- Boothroyd, A.T. Principles of Neutron Scattering from Condensed Matter; Oxford University Press: Oxford, UK, 2020; ISBN 9780198862314. [Google Scholar]
- Bethe, H. Zur Theorie Des Durchgangs Schneller Korpuskularstrahlen Durch Materie. Ann. Phys. 1930, 397, 325–400. [Google Scholar] [CrossRef]
- Beni, M.S.; Krstic, D.; Nikezic, D.; Yu, K.N. Monte Carlo Studies on Photon Interactions in Radiobiological Experiments. PLoS ONE 2018, 13, e0193575. [Google Scholar] [CrossRef] [Green Version]
- Haberland, H. Clusters of Atoms and Molecules; Haberland, H., Ed.; Springer Series in Chemical Physics; Springer: Berlin/Heidelberg, Germany, 1994; Volume 52, ISBN 9788578110796. [Google Scholar]
- Vaidya, J.S. Principles of Cancer Treatment by Radiotherapy. Surgery 2021, 39, 193–201. [Google Scholar] [CrossRef]
- Baldacchino, G.; Brun, E.; Denden, I.; Bouhadoun, S.; Roux, R.; Khodja, H.; Sicard-Roselli, C. Importance of Radiolytic Reactions during High-LET Irradiation Modalities: LET Effect, Role of O2 and Radiosensitization by Nanoparticles. Cancer Nanotechnol. 2019, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- McAuley, K.B. The Chemistry and Physics of Polyacrylamide Gel Dosimeters: Why They Do and Don t Work. J. Phys. Conf. Ser. 2004, 3, 29–33. [Google Scholar] [CrossRef]
- Fuxman, A.M.; McAuley, K.B.; Schreiner, L.J. Modeling of Free-Radical Crosslinking Copolymerization of Acrylamide and N,N′-Methylenebis(Acrylamide) for Radiation Dosimetry. Macromol. Theory Simul. 2003, 12, 647–662. [Google Scholar] [CrossRef]
- Fuxman, A.M.; McAuley, K.B.; Schreiner, L.J. Modelling of Polyacrylamide Gel Dosimeters with Spatially Non-Uniform Radiation Dose Distributions. Chem. Eng. Sci. 2005, 60, 1277–1293. [Google Scholar] [CrossRef]
- Koeva, V.I.; Daneshvar, S.; Senden, R.J.; Imam, A.H.M.; Schreiner, L.J.; McAuley, K.B. Mathematical Modeling of PAG- and NIPAM-Based Polymer Gel Dosimeters Contaminated by Oxygen and Inhibitor. Macromol. Theory Simul. 2009, 18, 495–510. [Google Scholar] [CrossRef]
- Chain, J.N.M.; Nasr, A.T.; Schreiner, L.J.; McAuley, K.B. Mathematical Modeling of Depth-Dose Response of Polymer-Gel Dosimeters. Macromol. Theory Simul. 2011, 20, 735–751. [Google Scholar] [CrossRef]
- Nasr, A.T.; Schreiner, L.J.; McAuley, K.B. Mathematical Modeling of the Response of Polymer Gel Dosimeters to HDR and LDR Brachytherapy Radiation. Macromol. Theory Simul. 2012, 21, 36–51. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. An Apparent Threshold Dose Response in Ferrous Xylenol-Orange Gel Dosimeters When Scanned with a Yellow Light Source. Phys. Med. Biol. 2008, 53, 1637–1650. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hsieh, L.L.; Chang, Y.J.; Wang, T.H.; Hsieh, B.T. Characterization of the Chemical Stability of Irradiated N-Isopropylacrylamide Gel Dosimeter. Radiat. Phys. Chem. 2013, 89, 76–82. [Google Scholar] [CrossRef]
- Mattea, F.; Chacón, D.; Vedelago, J.; Valente, M.; Strumia, M.C. Polymer Gel Dosimeter Based on Itaconic Acid. Appl. Radiat. Isot. 2015, 105, 98–104. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y. On the Accuracy and Precision of Gel Dosimetry. J. Phys. Conf. Ser. 2006, 56, 72–85. [Google Scholar] [CrossRef]
- Vergote, K.; De Deene, Y.; Vanden Bussche, E.; De Wagter, C. On the Relation between the Spatial Dose Integrity and the Temporal Instability of Polymer Gel Dosimeters. Phys. Med. Biol. 2004, 49, 4507–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibbott, G.S. Clinical Applications of Gel Dosimeters. J. Phys. Conf. Ser. 2006, 56, 108–131. [Google Scholar] [CrossRef]
- Bayreder, C.; Georg, D.; Moser, E.; Berg, A. Basic Investigations on the Performance of a Normoxic Polymer Gel with Tetrakis-Hydroxy-Methyl-Phosphonium Chloride as an Oxygen Scavenger: Reproducibility, Accuracy, Stability, and Dose Rate Dependence. Med. Phys. 2006, 33, 2506–2518. [Google Scholar] [CrossRef]
- De Deene, Y.; Hanselaer, P.; De Wagter, C.; Achten, E.; De Neve, W. An Investigation of the Chemical Stability of a Monomer/Polymer Gel Dosimeter. Phys. Med. Biol. 2000, 45, 859–878. [Google Scholar] [CrossRef]
- Olsson, L.E.; Petersson, S.; Ahlgren, L.; Mattsson, S. Ferrous Sulphate Gels for Determination of Absorbed Dose Distributions Using MRI Technique: Basic Studies. Phys. Med. Biol. 1989, 34, 43–52. [Google Scholar] [CrossRef]
- Ibbott, G.S. Applications of Gel Dosimetry. J. Phys. Conf. Ser. 2004, 3, 58–77. [Google Scholar] [CrossRef] [Green Version]
- Gambarini, G.; Arrigoni, S.; Cantone, M.C.; Molho, N.; Facchielli, L.; Sichirollo, A.E. Dose-Response Curve Slope Improvement and Result Reproducibility of Ferrous-Sulphate-Doped Gels Analysed by NMR Imaging. Phys. Med. Biol. 1994, 39, 703–717. [Google Scholar] [CrossRef]
- Cosgrove, V.P.; Murphy, P.S.; McJury, M.; Adams, E.J.; Warrington, A.P.; Leach, M.O.; Webb, S. The Reproducibility of Polyacrylamide Gel Dosimetry Applied to Stereotactic Conformal Radiotherapy. Phys. Med. Biol. 2000, 45, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.; Chacón, D.; Mattea, F.; Meilij, R.; Pérez, P.; Romero, M.; Scarinci, I.; Vedelago, J.; Vitullo, F.; Wolfel, A. Linear Energy Transfer Characterization of Five Gel Dosimeter Formulations for Electron and Proton Therapeutic Beams. Appl. Radiat. Isot. 2021, 178, 109972. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Wang, Y.; Bi, Z.; Xue, S.; Ou, B.; Liu, K. Intraoperative Radiotherapy (IORT) versus Whole-Breast External Beam Radiotherapy (EBRT) in Early Stage Breast Cancer: Results from SEER Database. Jpn. J. Radiol. 2020, 38, 85–92. [Google Scholar] [CrossRef]
- Farajollahi, A.R.; Bonnett, D.E.; Ratcliffe, A.J.; Aukett, R.J.; Mills, J.A. An Investigation into the Use of Polymer Gel Dosimetry in Low Dose Rate Brachytherapy. Br. J. Radiol. 1999, 72, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Santibáñez, M.; Guillen, Y.; Chacón, D.; Figueroa, R.G.; Valente, M. Feasibility of Dose Enhancement Assessment: Preliminary Results by Means of Gd-Infused Polymer Gel Dosimeter and Monte Carlo Study. Appl. Radiat. Isot. 2018, 141, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Carrara, M.; Gambarini, G.; Borroni, M.; Tomatis, S.; Negri, A.; Pirola, L.; Cerrotta, A.; Fallai, C.; Zonca, G. Characterisation of a Fricke Gel Compound Adopted to Produce Dosimetric Catheters for in Vivo Dose Measurements in HDR Brachytherapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2011, 652, 888–890. [Google Scholar] [CrossRef]
- Valente, M.; Vedelago, J.; Chacón, D.; Mattea, F.; Velásquez, J.; Pérez, P. Water-Equivalence of Gel Dosimeters for Radiology Medical Imaging. Appl. Radiat. Isot. 2018, 141, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Šolc, J.; Sochor, V. Feasibility of Radiochromic Gels for 3D Dosimetry of Brachytherapy Sources. Metrologia 2012, 49, S231–S236. [Google Scholar] [CrossRef]
- Massillon-Jl, G.; Minniti, R.; Mitch, M.G.; Maryanski, M.J.; Soares, C.G. The Use of Gel Dosimetry to Measure the 3D Dose Distribution of a 90Sr/90Y Intravascular Brachytherapy Seed. Phys. Med. Biol. 2009, 54, 1661–1672. [Google Scholar] [CrossRef] [Green Version]
- Pantelis, E.; Karlis, A.K.; Kozicki, M.; Papagiannis, P.; Sakelliou, L.; Rosiak, J.M. Polymer Gel Water Equivalence and Relative Energy Response with Emphasis on Low Photon Energy Dosimetry in Brachytherapy. Phys. Med. Biol. 2004, 49, 3495–3514. [Google Scholar] [CrossRef]
- Venning, A.J.; Nitschke, K.N.; Keall, P.J.; Baldock, C. Radiological Properties of Normoxic Polymer Gel Dosimeters. Med. Phys. 2005, 32, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Antoniou, P.E.; Bousbouras, P.; Sandaltzopoulos, R.; Kaldoudi, E. Investigating the Potential of Polymer Gel Dosimetry for Interventional Radiology: First Results. Phys. Med. Biol. 2008, 53, N127–N136. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.C. Radiation Dosimetry Using Fricke-Infused Gels and Magnetic Resonance Imaging. Proc. Natl. Sci. Counc. Repub. China. Part B Life Sci. 2001, 25, 1–11. [Google Scholar]
- Kipouros, P.; Pappas, E.; Baras, P.; Hatzipanayoti, D.; Karaiskos, P.; Sakelliou, L.; Sandilos, P.; Seimenis, I. Wide Dynamic Dose Range of VIPAR Polymer Gel Dosimetry. Phys. Med. Biol. 2001, 46, 2143–2159. [Google Scholar] [CrossRef] [PubMed]
- Zehtabian, M.; Faghihi, R.; Zahmatkesh, M.H.; Meigooni, A.S.; Mosleh-Shirazi, M.A.; Mehdizadeh, S.; Sina, S.; Bagheri, S. Investigation of the Dose Rate Dependency of the PAGAT Gel Dosimeter at Low Dose Rates. Radiat. Meas. 2012, 47, 139–144. [Google Scholar] [CrossRef]
- Pappas, E.P.; Zoros, E.; Moutsatsos, A.; Peppa, V.; Zourari, K.; Karaiskos, P.; Papagiannis, P. On the Experimental Validation of Model-Based Dose Calculation Algorithms for 192Ir HDR Brachytherapy Treatment Planning. Phys. Med. Biol. 2017, 62, 4160–4182. [Google Scholar] [CrossRef] [PubMed]
- Massillon-Jl, G.; Minniti, R.; Mitch, M.G.; Soares, C.G.; Hearn, R.A. High-Resolution 3D Dose Distribution Measured for Two Low-Energy X-ray Brachytherapy Seeds: 125I and 103Pd. Radiat. Meas. 2011, 46, 238–243. [Google Scholar] [CrossRef]
- Maynard, E.; Hilts, M.; Heath, E.; Jirasek, A. Evaluation of Accuracy and Precision in Polymer Gel Dosimetry. Med. Phys. 2017, 44, 736–746. [Google Scholar] [CrossRef]
- Hill, B.; Venning, A.; Baldock, C. The Dose Response of Normoxic Polymer Gel Dosimeters Measured Using X-ray CT. Br. J. Radiol. 2005, 78, 623–630. [Google Scholar] [CrossRef]
- Chen, K.; Vyazovkin, S. Temperature Dependence of Sol-Gel Conversion Kinetics in Gelatin-Water System. Macromol. Biosci. 2009, 9, 383–392. [Google Scholar] [CrossRef]
- Romero, M.; Mattea, F.; Vedelago, J.; Chacón, D.; Valente, M.; Igarzabal, C.Á.; Strumia, M. Analytical and Rheological Studies of Modified Gel Dosimeters Exposed to X-ray Beams. Microchem. J. 2016, 127, 231–236. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Pastorello, B.F.; De Araujo, D.B.; Baffa, O. Formaldehyde Increases MAGIC Gel Dosimeter Melting Point and Sensitivity. Phys. Med. Biol. 2008, 53, N53–N58. [Google Scholar] [CrossRef]
- Chacón, D.; Strumia, M.; Valente, M.; Mattea, F. Effect of Inorganic Salts and Matrix Crosslinking on the Dose Response of Polymer Gel Dosimeters Based on Acrylamide. Radiat. Meas. 2018, 117, 7–18. [Google Scholar] [CrossRef]
- d’Errico, F.; Lazzeri, L.; Dondi, D.; Mariani, M.; Marrale, M.; Souza, S.O.; Gambarini, G. Novel GTA-PVA Fricke Gels for Three-Dimensional Dose Mapping in Radiotherapy. Radiat. Meas. 2017, 106, 612–617. [Google Scholar] [CrossRef]
- Gallo, S.; Gambarini, G.; Veronese, I.; Argentiere, S.; Gargano, M.; Ianni, L.; Lenardi, C.; Ludwig, N.; Pignoli, E.; d’Errico, F. Does the Gelation Temperature or the Sulfuric Acid Concentration Influence the Dosimetric Properties of Radiochromic PVA-GTA Xylenol Orange Fricke Gels? Radiat. Phys. Chem. 2019, 160, 35–40. [Google Scholar] [CrossRef]
- George, V.; Britto, I.J.; Sebastian, M.S. Studies on Radiation Grafting of Methyl Methacrylate onto Natural Rubber for Improving Modulus of Latex Film. Radiat. Phys. Chem. 2003, 66, 367–372. [Google Scholar] [CrossRef]
- Fuse, H.; Oyama, S.; Yasue, K.; Ito, S.; Sato, T.; Fujisaki, T.; Abe, S.; Oyama, K.; Suzuki, A.; Yoshizawa, T.; et al. Design and Characteristics of an Agar Additive Polymer Gel Dosimeter. Appl. Radiat. Isot. 2019, 151, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Maeyama, T.; Yasuhiro, I.; Yoshihiro, K.; Fukasaku, K.; Ishikawa, K.L.; Fukunishi, N. Polymer Gel Dosimeter with AQUAJOINT® as Hydrogel Matrix. Radiat. Phys. Chem. 2018, 146, 121–125. [Google Scholar] [CrossRef]
- Jaszczak, M.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Substituting Gelatine with Pluronic F-127 Matrix in 3D Polymer Gel Dosimeters Can Improve Nuclear Magnetic Resonance, Thermal and Optical Properties. Phys. Med. Biol. 2018, 63, 175010. [Google Scholar] [CrossRef]
- Hilts, M.; Jirasek, A.; Duzenli, C. Effects of Gel Composition on the Radiation Induced Density Change in PAG Polymer Gel Dosimeters: A Model and Experimental Investigations. Phys. Med. Biol. 2004, 49, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Koeva, V.I.; Csaszar, E.S.; Senden, R.J.; McAuley, K.B.; Schreiner, L.J. Polymer Gel Dosimeters with Increased Solubility: A Preliminary Investigation of the NMR and Optical Dose-Response Using Different Crosslinkers and Co-Solvents. Macromol. Symp. 2008, 261, 157–166. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M.; Cłapa, M. On the Development of a VIPAR Nd Radiotherapy 3D Polymer Gel Dosimeter. Phys. Med. Biol. 2017, 62, 986–1008. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Al-Ajaleen, M.S.; Abuzayed, M.H.; Aldweri, F.M.; Eyadeh, M.M. High Dose Sensitivity of N-(Isobutoxymethyl)Acrylamide Polymer Gel Dosimeters with Improved Monomer Solubility Using Acetone Co-Solvent. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 442, 67–72. [Google Scholar] [CrossRef]
- Song, T.; Tanpichai, S.; Oksman, K. Cross-Linked Polyvinyl Alcohol (PVA) Foams Reinforced with Cellulose Nanocrystals (CNCs). Cellulose 2016, 23, 1925–1938. [Google Scholar] [CrossRef]
- Banerjee, S.; Roy, S.; Bagchi, B. Enhanced Pair Hydrophobicity in the Water−Dimethylsulfoxide (DMSO) Binary Mixture at Low DMSO Concentrations. J. Phys. Chem. B 2010, 114, 12875–12882. [Google Scholar] [CrossRef]
- Sandrin, D.; Wagner, D.; Sitta, C.E.; Thoma, R.; Felekyan, S.; Hermes, H.E.; Janiak, C.; De Sousa Amadeu, N.; Kühnemuth, R.; Löwen, H.; et al. Diffusion of Macromolecules in a Polymer Hydrogel: From Microscopic to Macroscopic Scales. Phys. Chem. Chem. Phys. 2016, 18, 12860–12876. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y.; Venning, A.; Hurley, C.; Healy, B.J.; Baldock, C. Dose-Response Stability and Integrity of the Dose Distribution of Various Polymer Gel Dosimeters. Phys. Med. Biol. 2002, 47, 2459–2470. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Heilemann, G.; Kuess, P.; Georg, D.; Berg, A. The Impact of the Oxygen Scavenger on the Dose-Rate Dependence and Dose Sensitivity of MAGIC Type Polymer Gels. Phys. Med. Biol. 2018, 63, 06NT01. [Google Scholar] [CrossRef] [PubMed]
- Jirasek, A.; Hilts, M.; Shaw, C.; Baxter, P. Investigation of Tetrakis Hydroxymethyl Phosphonium Chloride as an Antioxidant for Use in X-ray Computed Tomography Polyacrylamide Gel Dosimetry. Phys. Med. Biol. 2006, 51, 1891–1906. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Basfar, A.A.; Almousa, A.A.; Devic, S.; Moftah, B. New Normoxic N-(Hydroxymethyl)Acrylamide Based Polymer Gel for 3D Dosimetry in Radiation Therapy. Phys. Med. 2017, 33, 121–126. [Google Scholar] [CrossRef]
- Jaszczak, M.; Kolesińska, B.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Examination of THPC as an Oxygen Scavenger Impacting VIC Dosimeter Thermal Stability and Comparison of NVP-Containing Polymer Gel Dosimeters. Phys. Med. Biol. 2019, 64, 035019. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Bujold, R.; Lepage, M. Preliminary Studies on the Role and Reactions of Tetrakis(Hydroxymethyl) Phosphonium Chloride in Polyacrylamide Gel Dosimeters. Phys. Med. Biol. 2012, 57, 5981–5994. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, M.; Bujold, R.; Lepage, M. Severe Dose Inaccuracies Caused by an Oxygen-Antioxidant Imbalance in Normoxic Polymer Gel Dosimeters. Phys. Med. Biol. 2011, 56, 601–625. [Google Scholar] [CrossRef]
- Magugliani, G.M.; Liosi, M.; Marranconi, M.; Micotti, E.; Caprioli, M.; Gambirasio, A.; Locatelli, F.; Macerata, E.; Mossini, E.; Salmoiraghi, P.; et al. Practical Role of Polymerization Inhibitors in Polymer Gel Dosimeters. Nuovo Cim. 2020, 43, 147. [Google Scholar]
- Mei, Q.; Cao, H.; Han, D.; Li, M.; Yao, S.; Xie, J.; Zhan, J.; Zhang, Q.; Wang, W.; He, M. Theoretical Insight into the Degradation of P-Nitrophenol by OH Radicals Synergized with Other Active Oxidants in Aqueous Solution. J. Hazard. Mater. 2020, 389, 121901. [Google Scholar] [CrossRef]
- Seniwal, B.; Mendes, B.M.; Malano, F.; Pérez, P.; Valente, M.; Fonseca, T.C.F. Monte Carlo Assessment of Low Energy Electron Range in Liquid Water and Dosimetry Effects. Phys. Med. 2020, 80, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Li, W.B.; Belchior, A.; Beuve, M.; Chen, Y.Z.; Di Maria, S.; Friedland, W.; Gervais, B.; Heide, B.; Hocine, N.; Ipatov, A.; et al. Intercomparison of Dose Enhancement Ratio and Secondary Electron Spectra for Gold Nanoparticles Irradiated by X-rays Calculated Using Multiple Monte Carlo Simulation Codes. Phys. Med. 2020, 69, 147–163. [Google Scholar] [CrossRef] [Green Version]
- Mattea, F.; Vedelago, J.; Malano, F.; Gomez, C.; Strumia, M.C.; Valente, M. Silver Nanoparticles in X-ray Biomedical Applications. Radiat. Phys. Chem. 2017, 130, 442–450. [Google Scholar] [CrossRef]
- Casta, R.; Champeaux, J.P.; Moretto-Capelle, P.; Sence, M.; Cafarelli, P. Electron and Photon Emissions from Gold Nanoparticles Irradiated by X-ray Photons. J. Nanopart. Res. 2015, 17, 3. [Google Scholar] [CrossRef]
- Kuncic, Z.; Lacombe, S. Nanoparticle Radio-Enhancement: Principles, Progress and Application to Cancer Treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef]
- Titus, D.; Samuel, E.J.J.; Mohana Roopan, S. Current Scenario of Biomedical Aspect of Metal-Based Nanoparticles on Gel Dosimetry. Appl. Microbiol. Biotechnol. 2016, 100, 4803–4816. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.; Riyahi Alam, N.; Haghgoo, S.; Khoobi, M.; Geraily, G.H.; Gorji, E. Dosimetry and Radioenhancement Comparison of Gold Nanoparticles in Kilovoltage and Megavoltage Radiotherapy Using MAGAT Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2019, 9, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.Y.; Kwon, S.-I.; Chun, J.; Shin, H.S.; Chang, S.K.; Im, J.H.; Lee, J. Il Effect of Gold Nanoparticles on Dose Enhancement in Brachytherapy Using a Polymer Gel Dosimeter. J. Korean Phys. Soc. 2019, 75, 415–423. [Google Scholar] [CrossRef]
- Behrouzkia, Z.; Zohdiaghdam, R.; Khalkhali, H.R.; Mousavi, F. Evaluation of Gold Nanoparticle Size Effect on Dose Enhancement Factor in Megavoltage Beam Radiotherapy Using Magica Polymer Gel Dosimeter. J. Biomed. Phys. Eng. 2019, 9, 89–96. [Google Scholar] [CrossRef]
- Lima, I.S.; Guidelli, E.J.; Baffa, O. Dose Enhancement Factor Caused by Gold Nanoparticles: Influence of the Dosimetric Sensitivity and Radiation Dose Assessed by Electron Spin Resonance Dosimetry. Phys. Med. Biol. 2021, 66, 215013. [Google Scholar] [CrossRef]
- Vedelago, J.; Gomez, C.G.; Valente, M.; Mattea, F. Green Synthesis of Silver Nanoparticles Aimed at Improving Theranostics. Radiat. Phys. Chem. 2018, 146, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Vedelago, J.; Mattea, F.; Valente, M. Integration of Fricke Gel Dosimetry with Ag Nanoparticles for Experimental Dose Enhancement Determination in Theranostics. Appl. Radiat. Isot. 2018, 141, 182–186. [Google Scholar] [CrossRef]
- Merkis, M.; Urbonavicius, B.G.; Adliene, D.; Laurikaitiene, J.; Puiso, J. Pilot Study of Polymerization Dynamics in NMAG Dose Gel. Gels 2022, 8, 288. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Tadros, S.M.; Beshir, W.B.; Saad, G.R.; Gallo, S.; Ali, L.I.; Naoum, M.M. Study of Ag Nanoparticles in a Polyacrylamide Hydrogel Dosimeters by Optical Technique. Gels 2022, 8, 222. [Google Scholar] [CrossRef]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-Controlled Synthesis of Silver Nanoparticles for Plasmonic and Sensing Applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Merkis, M.; Puišo, J.; Adliene, D.; Laurikaitiene, J. Development and Characterization of Silver Containing Free Standing Polymer FILMS for Dosimetry Applications. Polymers 2021, 13, 3925. [Google Scholar] [CrossRef]
- Santibáñez, M.; Fuentealba, M.; Vedelago, J.; Chacón, D.; Mattea, F.; Valente, M. Experimental Characterization and Monte Carlo Simulations of the Dose Enhancement on the Millimeter Scale of PAGAT Infused with Gadolinium. Radiat. Phys. Chem. 2021, 186, 109533. [Google Scholar] [CrossRef]
- Farahani, S.; Riyahi Alam, N.; Haghgoo, S.; Shirazi, A.; Geraily, G.; Gorji, E.; Kavousi, N. The Effect of Bismuth Nanoparticles in Kilovoltage and Megavoltage Radiation Therapy Using Magnetic Resonance Imaging Polymer Gel Dosimetry. Radiat. Phys. Chem. 2020, 170, 108573. [Google Scholar] [CrossRef]
- Rajaee, A.; Wang, S.; Zhao, L.; Wang, D.; Liu, Y.; Wang, J.; Ying, K. Multifunction Bismuth Gadolinium Oxide Nanoparticles as Radiosensitizer in Radiation Therapy and Imaging. Phys. Med. Biol. 2019, 64, 195007. [Google Scholar] [CrossRef] [PubMed]
- Manohar, N.; Reynoso, F.J.; Diagaradjane, P.; Krishnan, S.; Cho, S.H. Quantitative Imaging of Gold Nanoparticle Distribution in a Tumor-Bearing Mouse Using Benchtop X-ray Fluorescence Computed Tomography. Sci. Rep. 2016, 6, 22079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, B.L.; Krishnan, S.; Cho, S.H. Estimation of Microscopic Dose Enhancement Factor around Gold Nanoparticles by Monte Carlo Calculations. Med. Phys. 2010, 37, 3809–3816. [Google Scholar] [CrossRef] [PubMed]







| Author and Year | Gel Type | Beam Type | Treatment Type | Technique Type | Readout System |
|---|---|---|---|---|---|
| Yao et al. 2019 [36] | NIPAM | Photon | External | IMRT and VMAT | Optical CT |
| Elter et al., 2019 [37] | PAGAT | Photon | External | MRgRT | MRI |
| Hillbrand et al., 2019 [38] | VIP | Proton | External | Pencil beam | MRI |
| Watanabe et al., 2019 [39] | VIPET | 192Ir | Brachytherapy | - | MRI |
| Abtahi et al., 2020 [40] | NIPAM | Photon | External | Intraoperative radiotherapy | MRI |
| Chou et al., 2020 [41] | NIPAM | Photon | External | IGRT | Optical CT |
| Kozicki et al., 2020 [42] | VIC and VIC-T | Photon | External | Stereotactic radiosurgery | MRI |
| Pant et al., 2020 [43] | NIPAM | Photon | External | Stereotactic radiosurgery | CBCT |
| Mann et al., 2020 [44] | PAGAT | Photon | External | IGRT | MRI |
| Schwahofer et al., 2020 [45] | PAGAT | Photon | External | MRgRT | MRI |
| Elter et al., 2021 [46] | PAGAT | Photon | External | MRgRT | MRI |
| Nezhad et al., 2021 [47] | MAGAT | Photon | External | Intraoperative radiotherapy | MRI |
| Azadeh et al., 2022 [48] | MAGIC-f | Photons | External | Stereotactic radiosurgery | MRI |
| Fuse et al., 2022 [49] | PAGAT-MgCl2 | Photon | External | IMRT | MRI |
| Kim et al., 2022 [50] | MAGAT | Photon | External | MR-Linac (isocenter verification) | MRI |
| Kudrevicius et al., 2022 [51] | nPAG | Photon | External | Gamma knife | MRI |
| Watanabe et al., 2022 [52] | VIPET | 192 Ir and photon | Brachytherapy and external | - IMRT | MRI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macchione, M.A.; Lechón Páez, S.; Strumia, M.C.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. https://doi.org/10.3390/gels8100663
Macchione MA, Lechón Páez S, Strumia MC, Valente M, Mattea F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels. 2022; 8(10):663. https://doi.org/10.3390/gels8100663
Chicago/Turabian StyleMacchione, Micaela A., Sofía Lechón Páez, Miriam C. Strumia, Mauro Valente, and Facundo Mattea. 2022. "Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review" Gels 8, no. 10: 663. https://doi.org/10.3390/gels8100663
APA StyleMacchione, M. A., Lechón Páez, S., Strumia, M. C., Valente, M., & Mattea, F. (2022). Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels, 8(10), 663. https://doi.org/10.3390/gels8100663