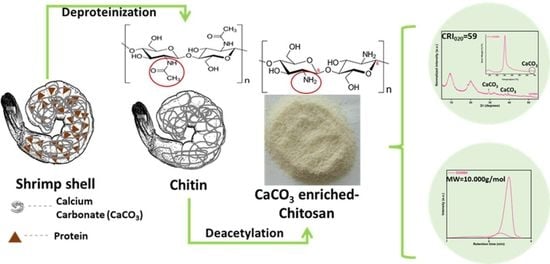
A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chitosan Synthesis from Commercial Chitin
2.1.1. FTIR Structural Analysis of CCHC Series
2.1.2. Deacetylation Degree and Molecular Weight Distribution of CCHC Series
2.2. Synthesis and Characterization of Chitin and Chitosan from Shrimp Shells Waste
2.2.1. FTIR Spectroscopy of CCHSH and Intermediaries
2.2.2. Deacetylation Degree and Molecular Weight Distribution of CCHSH
2.2.3. XRD Patterns of CCHSH and Intermediaries
2.2.4. Thermogravimetric Profiles (TGA/DTG) of CCHSH and Intermediaries
2.2.5. N2 Adsorption/Desorption Measurements and Morphology of CCHSH and Intermediates
2.2.6. Rheological Properties of CCHSH
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Chitosan
4.2.1. Synthesis of Chitosan from Commercial Chitin
4.2.2. Isolation of Chitin and Synthesis of Chitosan from Shrimp Shell Waste
4.3. Determination of Deacetylation Degree (DD)
4.4. Characterization Techniques
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, B.; Malik, P.; Jain, P. Biopolymer reinforced nanocomposites: A comprehensive review. Mater. Today Commun. 2018, 16, 353–363. [Google Scholar] [CrossRef]
- Nandakumar, A.; Chuah, J.-A.; Sudesh, K. Bioplastics: A boon or bane? Renew. Sustain. Energy Rev. 2021, 147, 111237. [Google Scholar] [CrossRef]
- Tokatlı, K.; Demirdöven, A. Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food Process. Preserv. 2017, 42, e13494. [Google Scholar] [CrossRef]
- Jacob, J.; Gopi, S. Chapter 3—Isolation and PhysicoChemical Characterization of Biopolymers. In Biopolymers and Their Industrial Applications Biopolymers and Their Industrial Applications. From Plant, Animal, and Marine Sources, to Functional Products, 1st ed.; Thomas, S., Gopi, S., Amalraj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 45–79. [Google Scholar] [CrossRef]
- Sandeep, A.; Sangameshwar, K.; Mukesh, G.; Chandrakant, R.; Avinash, D. A brief overview on chitosan applications. IAJPR 2013, 3, 2231–6876. [Google Scholar]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.; Franco, L.O.; Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef]
- Al Shaqsi, N.H.K.; Al Hoqani, H.A.S.; Hossain, M.A.; Al Sibani, M.A. Isolation, characterization and standardization of demineralization process for chitin polymer and minerals from the crabs waste of Portunidae segnis. ABST 2020, 2, 45–58. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Adegbite, A.A.; Akinwande, C. Organic and mineral acid demineralizations: Effects on crangon and Liocarcinus vernalis—Sourced biopolymer yield and properties. J. Taibah Univ. Sci. 2018, 12, 837–845. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Heras, A. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Sivashankari, P.R.; Prabaharan, M. Deacetylation modification techniques of chitin and chitosan. Chitosan Based Biomater. 2017, 1, 117–133. [Google Scholar] [CrossRef]
- Crini, N.M.; Lichtfouse, E.; Torri, G.; Crini, G. Fundamentals and Applications of Chitosan in Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2019; pp. 49–123. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.-J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Hu, X.; Tian, Z.; Li, X.; Wang, S.; Pei, H.; Sun, H.; Zhang, Z. Green, Simple, and Effective Process for the Comprehensive Utilization of Shrimp Shell Wast. ACS Omega 2020, 5, 19227–19235. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, S.; Siyamak, S.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Rahman, M.Z.A.; Azizi, S.; Fatehi, A. Enhancement of Mechanical and Thermal Properties of Polycaprolactone/Chitosan Blend by Calcium Carbonate Nanoparticles. Int. J. Mol. Sci. 2012, 13, 4508–4522. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lim, J.K.; Park, J.S. Enhancement of Mechanical Stability and Ionic Conductivity of Chitosan-based Solid Polymer Electrolytes Using Silver Nanowires as Fillers. BKCS 2019, 40, 898–905. [Google Scholar] [CrossRef]
- Mesgar, A.S.; Mohammadi, Z.; Khosrovan, S. Improvement of mechanical properties and in vitro bioactivity of freeze-dried gelatin/chitosan scaffolds by functionalized carbon nanotubes. Int. J. Polym. Mater. 2017, 67, 267–276. [Google Scholar] [CrossRef]
- Koumentakou, I.; Terzopoulou, Z.; Michopoulou, A.; Kalafatakis, I.; Theodorakis, K.; Tzetzis, D.; Bikiaris, D. Chitosan dressings containing inorganic additives and levofloxacin as potential wound care products with enhanced hemostatic properties. Int. J. Biol. Macromol. 2020, 162, 693–703. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crops. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Kelberg, E.A.; Evmenenko, G.A.; Bronnikov, S.V. Improvement of the mechanical properties of chitosan films by the addition of poly(ethylene oxide). Polym. Eng. Sci. 2000, 40, 1211–1215. [Google Scholar] [CrossRef]
- Phung Hai, T.A.; Sugimoto, R. Fluorescence control of chitin and chitosan fabricated via surface functionalization using direct oxidative polymerization. RSC Adv. 2014, 8, 7005–7013. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The role of chitin-rich skeletal organic matrix on the crystallization of calcium carbonate in the crustose coralline alga Leptophytum foecundum. Sci Rep. 2019, 9, 11869. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Huang, X.; Zhang, J.; Zhu, Y.; Liu, Y.; Liu, B.; Wang, Q.; Huang, X.; He, D. CaCO3–Chitosan Composites Granules for Instant Hemostasis and Wound Healing. Materials 2021, 14, 3350. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. In Solubility of Polysaccharides; Xu, Z., Ed.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Mekahlia, S.; Bouzid, B. Chitosan-Copper (II) complex as antibacterial agent: Synthesis, characterization and coordinating bond- activity correlation study. Phys. Procedia 2009, 2, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, F.M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2014, 13, 141–158. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab shells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Nemtsev, S.V.; Gamzazade, A.I.; Rogozhin, S.V.; Bykova, V.M.; Bykov, V.P. Deacetylation of chitin under homogeneous conditions. Appl. Biochem. Microbiol. 2002, 38, 521–526. [Google Scholar] [CrossRef]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S.; Olaleye, S.A. Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. JESTECH 2017, 20, 1155–1165. [Google Scholar] [CrossRef]
- Hajji, S.; Turki, T.; Boubakri, A.; Ben Amor, M.; Mzoughi, N. Study of cadmium adsorption onto calcite using full factorial experiment design. Desalination Water Treat. 2017, 83, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Rane, S.S.; Choi, P. Polydispersity Index: How Accurately Does It Measure the Breadth of the Molecular Weight Distribution? Chem. Mater. 2005, 17, 926. [Google Scholar] [CrossRef]
- Ambarish, C.A.; Sridhar, K.R. Isolation and Characterization of Chitin from Exoskeleton of Pill-Millipedes. Trends Biomater. Artif. Organs 2015, 29, 155–159. Available online: https://biomaterials.org.in/tibao/index.php/tibao/article/view/142 (accessed on 14 October 2022).
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Baez, C.; Verrill, S.P. Effect of sample moisture content on XRD-estimated cellulose crystallinity index and crystallite size. Cellulose 2017, 24, 1971–1984. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of Spectroscopic Methods for Structural Analysis of Chitin and Chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- ALOthman, Z. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef] [Green Version]
- Bradić, B.; Bajec, D.; Pohar, A.; Novak, U.; Likozara, B. A reaction-diffusion kinetic model for the heterogeneous N-deacetylation step in chitin material conversion to chitosan in catalytic alkaline solutions. React. Chem. Eng. 2018, 6, 920–929. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Elgannoudi, E.S.; Mainal, A.; Yahaya, A.H. Characterization of chitosan in acetic acid: Rheological and thermal studies. Turk. J. Chem. 2010, 34, 47–56. [Google Scholar] [CrossRef]
- Desbrieres, J. Viscosity of semiflexible chitosan solutions: Influence of concentration, temperature, and role of intermolecular interactions. Biomacromolecules 2002, 3, 342–349. [Google Scholar] [CrossRef]
- El-Hefian, E.A.; Yahaya, A.H. Rheological study of chitosan and its blends: An overview. Maejo Int. J. Sci. Technol. 2010, 4, 210–220. [Google Scholar]
- Dresvyanina, E.; Yudenko, A.; Lebedeva, I.; Popryadukhin, P.; Dobrovolskaya, I.; Yudin, V.; Morganti, P. Comparison of electrospinning and wet-spinning methods for the production of chitosan-based composite fibers. Mater. Technol. 2018, 52, 39–42. [Google Scholar] [CrossRef]
- Martínez-Ruvalcaba, A.; Chornet, E.; Rodrigue, D. Dynamic Rheological Properties of Concentrated Chitosan Solutions. Appl. Rheol. 2004, 14, 140–147. [Google Scholar] [CrossRef]







| Sample | DD% a | Mn (g/mol) | PIb | Area (%) | |||
|---|---|---|---|---|---|---|---|
| (Peak 1) | (Peak 2) | (Peak 1) | (Peak 2) | (Peak 1) | (Peak 2) | ||
| CC | 79.00 | 2.045 × 105 | - | 2.39 | - | 100 | - |
| CCHC4 | 70.95 | 6.558 × 105 | 1.062 × 104 | 19.47 | 1.11 | 90.1 | 9.9 |
| CCHC4(2) | 74.57 | 7.837 × 105 | 1.224 × 104 | 22.53 | 1.02 | 98.1 | 1.9 |
| CCHC6 | 78.19 | 5.729 × 105 | 1.158 × 104 | 26.26 | 1.09 | 92.7 | 7.3 |
| CCHSH | 75.38 | 1.655 × 106 | 9.059 × 103 | 6.19 | 1.39 | 29.7 | 70.3 |
| Sample | CRI020 | CRI110 | Total Mass Loss (%) | Tmax (°C) | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| SH | 55.55 | 55.55 | 65.57 | 56.18 | 300 (shoulder)/352.93 | 662.59 |
| CHSH | 61.26 | 57.00 | 76.64 | 53.91 | 362.08 | 678.34 |
| CHC | 71.23 | 78.78 | 99.50 | 33.4 | 367.46 | - |
| CC | 37.03 | 66.00 | 68.93 | 44.57 | 287.84 | - |
| CCHC6 | 53.08 | 62.00 | 68.35 | 44.82 | 288.38 | - |
| CCHSH | 59.42 | 44.55 | 55.02 | 45.77 | 305.40 | 691.92 |
| Sample | BET Surface Area (m2 g−1) | Pore Surface Area (m2 g−1) | Maximum Pore Diameter a (nm) | Micropore Volume b (cm3 g−1) |
|---|---|---|---|---|
| SH | 3.364 | 3.989 | 4.15 | 0.0051 |
| CHSH | 9.805 | 13.860 | 3.97 | 0.0224 |
| CHC | 6.875 | 7.657 | 3.17 | 0.0142 |
| CC | 0.279 | 0.899 | 4.54 | 0.0011 |
| CCHSH | 7.817 | 11.16 | 4.15 | 0.0415 |
| CCHC6 | 2.164 | 2.436 | 4.54 | 0.0030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, A.; Sarbu, A.; Zaharia, A.; Sandu, T.; Iovu, H.; Fierascu, R.C.; Neagu, A.-L.; Chiriac, A.-L.; Iordache, T.-V. A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes. Gels 2022, 8, 742. https://doi.org/10.3390/gels8110742
Miron A, Sarbu A, Zaharia A, Sandu T, Iovu H, Fierascu RC, Neagu A-L, Chiriac A-L, Iordache T-V. A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes. Gels. 2022; 8(11):742. https://doi.org/10.3390/gels8110742
Chicago/Turabian StyleMiron, Andreea, Andrei Sarbu, Anamaria Zaharia, Teodor Sandu, Horia Iovu, Radu Claudiu Fierascu, Ana-Lorena Neagu, Anita-Laura Chiriac, and Tanta-Verona Iordache. 2022. "A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes" Gels 8, no. 11: 742. https://doi.org/10.3390/gels8110742
APA StyleMiron, A., Sarbu, A., Zaharia, A., Sandu, T., Iovu, H., Fierascu, R. C., Neagu, A. -L., Chiriac, A. -L., & Iordache, T. -V. (2022). A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes. Gels, 8(11), 742. https://doi.org/10.3390/gels8110742