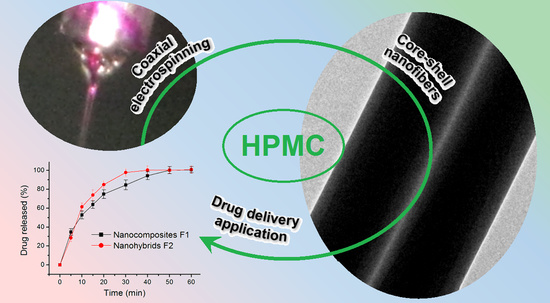
Electrospun Core (HPMC–Acetaminophen)–Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Two Different Working Processes from the Same Apparatus
2.2. The Morphologies and Inner Structure of the Nanofibers
2.3. The Physical Forms of Components and Their Compatibility
2.4. The Hydrophilic Properties
2.5. The In Vitro Drug Release Profiles and the Mechanisms
2.6. The Mechanism of the Influence of Shell PVP on the Gelatin of Core-Medicated HPMC
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparations
4.3. Characterizations
4.3.1. Morphologies and Inner Structures
4.3.2. Physical Forms and Compatibility
4.3.3. Properties
4.3.4. Functional Performances
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madaghiele, M.; Demitri, C.; Surano, I.; Silvestri, A.; Vitale, M.; Panteca, E.; Zohar, Y.; Rescigno, M.; Sannino, A. Biomimetic Cellulose-Based Superabsorbent Hydrogels for Treating Obesity. Sci. Rep. 2021, 11, 21394. [Google Scholar] [CrossRef]
- Bonetti, L.; De Nardo, L.; Farè, S. Chemically Crosslinked Methylcellulose Substrates for Cell Sheet Engineering. Gels 2021, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Gallo Stampino, P.; Riva, L.; Punta, C.; Elegir, G.; Bussini, D.; Dotelli, G. Comparative Life Cycle Assessment of Cellulose Nanofibres Production Routes from Virgin and Recycled Raw Materials. Molecules 2021, 26, 2558. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, D.; Song, X.; Zhao, Y.; Wang, Y.; Rao, L.; Fu, L.; Wang, Z.; Yang, X.; Li, Y.; et al. Recent Progress of Cellulose-Based Hydrogel Photocatalysts and Their Applications. Gels 2022, 8, 270. [Google Scholar] [CrossRef]
- Gebeyehu, E.K.; Sui, X.; Adamu, B.F.; Beyene, K.A.; Tadesse, M.G. Cellulosic-Based Conductive Hydrogels for Electro-Active Tissues: A Review Summary. Gels 2022, 8, 140. [Google Scholar] [CrossRef]
- Riva, L.; Fiorati, A.; Punta, C. Synthesis and Application of Cellulose-Polyethyleneimine Composites and Nanocomposites: A Concise Review. Materials 2021, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.; Fiorati, A.; D’Agostino, A.; Pelacani, C.M.; Chiesa, R.; Farè, S.; De Nardo, L. Smart Methylcellulose Hydrogels for PH-Triggered Delivery of Silver Nanoparticles. Gels 2022, 8, 298. [Google Scholar] [CrossRef]
- Jo, C.; Kim, S.S.; Rukmanikrishnan, B.; Ramalingam, S.; Prabakaran, D.S.; Lee, J. Properties of Cellulose Pulp and Polyurethane Composite Films Fabricated with Curcumin by Using NMMO Ionic Liquid. Gels 2022, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Hao, J. Identifying New Pathways and Targets for Wound Healing and Therapeutics from Natural Sources. Curr. Drug Deliv. 2021, 18, 1064–1084. [Google Scholar] [CrossRef]
- Park, K.; Choi, H.; Kang, K.; Shin, M.; Son, D. Soft Stretchable Conductive Carboxymethylcellulose Hydrogels for Wearable Sensors. Gels 2022, 8, 92. [Google Scholar] [CrossRef]
- Barba, A.A.; d’Amore, M.; Chirico, S.; Lamberti, G.; Titomanlio, G. Swelling of cellulose derivative (HPMC) matrix systems for drug delivery. Carbohydr. Polym. 2009, 78, 469–474. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun Tri-Layer Nanodepots for Sustained Release of Acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold Nanoparticles-Loaded Polyvinylpyrrolidone/Ethylcellulose Coaxial Electrospun Nanofibers with Enhanced Osteogenic Capability for Bone Tissue Regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Ghosal, K.; Augustine, R.; Zaszczynska, A.; Barman, M.; Jain, A.; Hasan, A.; Kalarikkal, N.; Sajkiewicz, P.; Thomas, S. Novel Drug Delivery Systems Based on Triaxial Electrospinning Based Nanofibers. React. Funct. Polym. 2021, 163, 104895. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Zhang, Z.; Bian, H.; Yong, Q. Procuring the Nano-Scale Lignin in Prehydrolyzate as Ingredient to Prepare Cellulose Nanofibril Composite Film with Multiple Functions. Cellulose 2020, 27, 9355–9370. [Google Scholar] [CrossRef]
- Araujo, R.; Soares, M.; Mazzei, J.L.; Ramos, M.; Siani, A.C. A Comparative Study of Hard Gelatin and Hypromellose Capsules Containing a Dry Extract of Senna (Cassia Angustifolia) under Controlled Temperature and Relative Humidity. Indian J. Pharm. Sci. 2021, 82, 718–723. [Google Scholar] [CrossRef]
- Khalid, G.M.; Selmin, F.; Musazzi, U.M.; Gennari, C.G.M.; Minghetti, P.; Cilurzo, F. Trends in the Characterization Methods of Orodispersible Films. Curr. Drug Deliv. 2021, 18, 941–952. [Google Scholar] [CrossRef]
- Ortega, C.A.; Favier, L.S.; Cianchino, V.A.; Cifuente, D.A. New Orodispersible Mini Tablets of Enalapril Maleate by Direct Compression for Pediatric Patients. Curr. Drug Deliv. 2020, 17, 505–510. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered Spindles of Little Molecules Around Electrospun Nanofibers for Biphasic Drug Release. Adv. Fiber Mater. 2022, 4, 305–317. [Google Scholar] [CrossRef]
- Yu, D.-G. Preface-Bettering Drug Delivery Knowledge from Pharmaceutical Techniques and Excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X.; et al. Transcutaneous Tumor Vaccination Combined with Anti-Programmed Death-1 Monoclonal Antibody Treatment Produces a Synergistic Antitumor Effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of Hollow Polymeric Nanosphere-Supported Imidazolium-Based Ionic Liquids with Enhanced Antimicrobial Activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Kose, M.D.; Ungun, N.; Bayraktar, O. Eggshell Membranebased Turmeric Extract Loaded Orally Disintegrating Films. Curr. Drug Deliv. 2021, 18, 547–559. [Google Scholar] [CrossRef]
- Verma, N.; Tiwari, A.; Bajpai, J.; Bajpai, A.K. Swelling Triggered Release of Cisplatin from Gelatin Coated Gold Nanoparticles. Inorg. Nano-Met. Chem. 2022, 52, 1–13. [Google Scholar] [CrossRef]
- Sultana, M.; Sultana, S.; Hussain, K.; Saeed, T.; Butt, M.A.; Raza, S.A.; Mahmood, R.; Hassan, S.; Anwer, U.U.; Bukhari, N.I. Enhanced Mefenamic Acid Release from Poloxamer-Silicon Dioxide Gel Filled in Hard Gelatin Capsules—An Application of Liquid Semisolid Matrix Technology for Insoluble. Curr. Drug Deliv. 2021, 18, 801–811. [Google Scholar] [CrossRef]
- Yang, H.; Lan, X.; Xiong, Y. In Situ Growth of Zeolitic Imidazolate Framework-L in Macroporous PVA/CMC/PEG Composite Hydrogels with Synergistic Antibacterial and Rapid Hemostatic Functions for Wound Dressing. Gels 2022, 8, 279. [Google Scholar] [CrossRef]
- Esim, O.; Hascicek, C. Lipid-Coated Nanosized Drug Delivery Systems for an Effective Cancer Therapy. Curr. Drug Deliv. 2021, 18, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Ejeta, F.; Gabriel, T.; Joseph, N.M.; Belete, A. Formulation, Optimization and In Vitro Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate Using a Combination of Superdisintegrant and Subliming Agent. Curr. Drug Deliv. 2021, 19, 129–141. [Google Scholar] [CrossRef]
- Bigogno, E.R.; Soares, L.; Mews, M.H.R.; Zétola, M.; Bazzo, G.C.; Stulzer, H.K.; Pezzini, B.R. It Is Possible to Achieve Tablets with Good Tabletability from Solid Dispersions-The Case of the High Dose Drug Gemfibrozil. Curr. Drug Deliv. 2021, 18, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Guan, S.M.; Su, J.J.; Liang, J.H.; Cui, L.L.; Zhang, K. The Development of Hyaluronic Acids Used for Skin Tissue Regeneration. Curr. Drug Deliv. 2021, 18, 836–846. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Zhong, A.; Li, X.; Boni, B.O.O.; Ahmed, A.A.Q.; Souho, T.; Zheng, R.; Shi, D.; Lamboni, L.; Yang, G. Biodegradable and Injectable Poly(Vinyl Alcohol) Microspheres in Silk Sericin Based Hydrogel for the Controlled Release of Antimicrobials: Application to Deep Full Thickness Burn Wound Healing. Adv. Compos. Hybrid Mater. 2022, 5, 1–26. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D.-G. Electrospun Medical Sutures for Wound Healing: A Review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Pang, L.; Liu, Y.; Lin, Y.; Xiang, T.; Li, J.; Liao, S.; Jiang, Y. Astragalus Polysaccharides/PVA Nanofiber Membranes Containing Astragaloside IV-Loaded Liposomes and Their Potential Use for Wound Healing. Evid.-Based Complement. Alternat. Med. 2022, 2022, 9716271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-Core/PHBV-Shell Fibers to Eliminate Tailing Off for an Improved Sustained Release of Curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, R.; Raman, S. Recent Trends in Carbon Nanotubes Based Prostate Cancer Therapy: A Biomedical Hybrid for Diagnosis and Treatment. Curr. Drug Deliv. 2022, 19, 229–237. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.G.; Guo, Z. Electrospun Structural Nanohybrids Combining Three Composites for Fast Helicide Delivery. Adv. Compos. Hybrid Mater. 2022, 5. [Google Scholar] [CrossRef]
- Riva, L.; Lotito, A.D.; Punta, C.; Sacchetti, A. Zinc- and Copper-Loaded Nanosponges from Cellulose Nanofibers Hydrogels: New Heterogeneous Catalysts for the Synthesis of Aromatic Acetals. Gels 2022, 8, 54. [Google Scholar] [CrossRef]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified Tri–Axial Electrospun Functional Core-Shell Nanofibrous Membranes for Natural Photodegradation of Antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Liu, R.; Hou, L.; Yue, G.; Li, H.; Zhang, J.; Liu, J.; Miao, B.; Wang, N.; Bai, J.; Cui, Z.; et al. Progress of Fabrication and Applications of Electrospun Hierarchically Porous Nanofibers. Adv. Fiber Mater. 2022, 4, 1–27. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun Nanofiber Membranes for Air Filtration: A Review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef]
- Bukhary, H.; Williams, G.R.; Orlu, M. Fabrication of Electrospun Levodopa-Carbidopa Fixed-Dose Combinations. Adv. Fiber Mater. 2020, 2, 194–203. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Tan, Y.; Li, D.; Xu, G.; Yin, D.; Xiao, Y.; Xu, T.; Chen, X.; Zhu, X.; Shi, X. Negative Isolation of Circulating Tumor Cells Using a Microfluidic Platform Integrated with Streptavidin-Functionalized PLGA Nanofibers. Adv. Fiber Mater. 2021, 3, 192–202. [Google Scholar] [CrossRef]
- Chen, M.; Le, T.; Zhou, Y.; Kang, F.; Yang, Y. Ultra-High Rate and Ultra-Stable Lithium Storage Enabled by Pore and Defect Engineered Carbon Nanofibers. Electrochim. Acta 2022, 420, 140429. [Google Scholar] [CrossRef]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole Topically Immobilized Electrospun Nanofibrous Scaffold: Novel Secondary Intention Wound Healing Accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating Current Electrospinning: The Impacts of Various High-Voltage Signal Shapes and Frequencies on the Spinnability and Productivity of Polycaprolactone Nanofifibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Brimo, N.; Serdaroglu, D.C.; Uysal, B. Comparing Antibiotic Pastes with Electrospun Nanofibers as Modern Drug Delivery Systems for Regenerative Endodontics. Curr. Drug Deliv. 2021, 18, 7. [Google Scholar] [CrossRef]
- Ziyadi, H.; Baghali, M.; Bagherianfar, M.; Mehrali, F.; Faridi-Majidi, R. An Investigation of Factors Affecting the Electrospinning of Poly(Vinyl Alcohol)/Kefiran Composite Nanofibers. Adv. Compos. Hybrid Mater. 2021, 4, 768–779. [Google Scholar] [CrossRef]
- Song, Y.; Huang, H.; He, D.; Yang, M.; Wang, H.; Zhang, H.; Li, J.; Li, Y.; Wang, C. Gallic Acid/2-Hydroxypropyl-Β-Cyclodextrin Inclusion Complexes Electrospun Nanofibrous Webs: Fast Dissolution, Improved Aqueous Solubility and Antioxidant Property of Gallic Acid. Chem. Res. Chin. Univ. 2021, 37, 450–455. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Jiang, D.; Pei, Y.; Wang, Z.; Zhou, X.; Wu, J.; Mo, X.; Wang, H. Delivery of Mrna Vaccines and Anti-PDL1 Sirna through Non-Invasive Transcutaneous Route Effectively Inhibits Tumor Growth. Compos. Part B Eng. 2022, 233, 109648. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, J.; Lyu, S.; Lu, Z.; Li, T.; Yang, Y.; Li, B.; Han, H.; Wu, B.; Sun, H.; et al. Poly(Lactic Acid)/Artificially Cultured Diatom Frustules Nanofibrous Membranes with Fast and Controllable Degradation Rates for Air Filtration. Adv. Compos. Hybrid Mater. 2022, 5. [Google Scholar] [CrossRef]
- Garcia Cervantes, M.Y.; Han, L.; Kim, J.; Chitara, B.; Wymer, N.; Yan, F. N-Halamine-Decorated Electrospun Polyacrylonitrile Nanofibrous Membranes: Characterization and Antimicrobial Properties. React. Funct. Polym. 2021, 168, 105058. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Yang, D.; Nie, J.; Ma, G. Electrospun Core-Shell Fibrous 2D Scaffold with Biocompatible Poly (Glycerol Sebacate) and Poly-L-Lactic Acid for Wound Healing. Adv. Fiber Mater. 2020, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhang, M.; Lv, H.; Zhou, Y.; Yang, Y.; Yu, D.-G. Electrospun Polyacrylonitrile-Based Lace Nanostructures and Their Cu(II) Adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and Characterization of Chitosan-Polycaprolactone Core-Shell Nanofibers Containing Tetracycline Hydrochloride. Colloids Surf. A 2022, 636, 128163. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun Hierarchical Structural Films for Effective Wound Healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, K.; Yu, D.-G.; Zheng, X.; Huang, C. Advances in Biosensing and Environmental Monitoring Based on Electrospun Nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
- Jiang, S.; Schmalz, H.G.; Agarwal, S.; Greiner, A. Electrospinning of ABS Nanofibers and Their High Filtration Performance. Adv. Fiber Mater. 2020, 2, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D.-G. Polymer-Based Nanofiber-Nanoparticle Hybrids and Their Medical Applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Deng, J.; Ke, Q.; Li, X.; Ouyang, Y.; Huang, C.; Liu, X.; Qian, Y. Grooved Fibers: Preparation Principles through Electrospinning and Potential Applications. Adv. Fiber Mater. 2021, 3, 203–213. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.; Qin, Y.; Li, C. Functional Electrospun Nanocomposites for Efficient Oxygen Reduction Reaction. Chem. Res. Chin. Univ. 2021, 37, 379–393. [Google Scholar] [CrossRef]
- Yu, D.-G.; Lv, H. Preface-Striding into Nano Drug Delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Diaz-Guerrero, A.M.; Castillo-Miranda, C.A.; Peraza-Vazquez, H.; Morales-Cepeda, A.B.; Pena-Delgado, A.F.; Rivera-Armenta, J.L.; Castro-Guerrero, C.F. Modelling of Acetaminophen Release from Hydroxyethylcellulose/Polyacrylamide Hydrogel. Mater. Res. Express 2021, 8, 015310. [Google Scholar] [CrossRef]
- Gaurkhede, S.G.; Osipitan, O.O.; Dromgoole, G.; Spencer, S.A.; Di Pasqua, A.J.; Denga, J. 3D Printing and Dissolution Testing of Novel Capsule Shells for Use in Delivering Acetaminophen. J. Pharm. Sci. 2021, 110, 3829–3837. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible Membranes from a Modified Coaxial Electrospinning for Fast Dissolution of Diclofenac Sodium. Membrances 2021, 11, 802. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun Structural Hybrids of Acyclovir-Polyacrylonitrile at Acyclovir for Modifying Drug Release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-G.; Wang, M.; Ge, R. Strategies for Sustained Drug Release from Electrospun Multi-Layer Nanostructures. WIRES Nanomed. Nanobi. 2022, 14, e1772. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun Coaxial Fibers to Optimize the Release of Poorly Water-Soluble Drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, G.; Zhao, Y.; Zhou, M.; Zhong, A.; Sun, J. Promotion of Skin Regeneration through Co-Axial Electrospun Fibers Loaded with Basic Fibroblast Growth Factor. Adv. Compos. Hybrid Mater. 2022, 5, 1–15. [Google Scholar] [CrossRef]
- Silva, P.M.; Prieto, C.; Andrade, C.C.P.; Lagaron, J.M.; Pastrana, L.M.; Coimbra, M.A.; Vicente, A.A.; Cerqueira, M.A. Hydroxypropyl methylcellulose-based micro- and nanostructures for encapsulation of melanoidins: Effect of electrohydrodynamic processing variables on morphological and physicochemical properties. Int. J. Biol. Macromol. 2022, 202, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Jozo, M.; Simon, N.; Yi, L.; Moczo, J.; Pukanszky, B. Improved Release of a Drug with Poor Water Solubility by Using Electrospun Water-Soluble Polymers as Carriers. Pharmaceutics 2022, 14, 34. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.Y.; Yu, D.-G. Electrospun Hybrid Films for Fast and Convenient Delivery of Active Herbs Extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-Saving Electrospinning with a Concentric Teflon-Core Rod Spinneret to Create Medicated Nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef] [PubMed]
- Balusamy, B.; Celebioglu, A.; Senthamizhan, A.; Uyar, T. Progress in the design and development of “fast-dissolving” electrospun nanofibers based drug delivery systems—A systematic review. J. Control. Release 2020, 326, 482–509. [Google Scholar] [CrossRef]
- Anderson, B.D. Predicting Solubility/Miscibility in Amorphous Dispersions: It Is Time to Move Beyond Regular Solution Theories. J. Pharm. Sci. 2018, 107, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Vovko, A.D.; Hodzic, B.; Brec, T.; Hudovornik, G.; Vrecer, F. Influence of Formulation Factors, Process Parameters, and Selected Quality Attributes on Carvedilol Release from Roller-Compacted Hypromellose-Based Matrix Tablets. Pharmaceutics 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Maskova, E.; Kubova, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlidalova, E.; Turanek, J.; Masek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.H.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In Situ Ophthalmic Gel Forming Systems of Poloxamer 407 and Hydroxypropyl Methyl Cellulose Mixtures for Sustained Ocular Delivery of Chloramphenicole: Optimization Study by Factorial Design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef]
- Peppas, N. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(A-L-Glutamic Acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A.H. Orodispersible films—Recent developments and new applications in drug delivery and therapy. Biochem. Pharmacol. 2022, 200, 115036. [Google Scholar] [CrossRef] [PubMed]
- Wiedey, R.; Kokott, M.; Breitkreutz, J. Orodispersible tablets for pediatric drug delivery: Current challenges and recent advances. Expert Opin. Drug Deliv. 2021, 18, 1873–1890. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid Films Prepared from a Combination of Electrospinning and Casting for Offering a Dual-Phase Drug Release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Wang, M.; Yu, D.-G.; Williams, G.R.; Bligh, S.W.A. Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect. Pharmaceutics 2022, 14, 1208. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, P.; Yang, Y.; Yu, D.G. Electrospun Beads-on-the-string Nanoproducts: Preparation and Drug Delivery Application. Curr. Drug Deliv. 2022, 19. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, M.; Song, W.; Zhang, Y.; Yu, D.-G.; Liu, Y. Electrospun Core (HPMC–Acetaminophen)–Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery. Gels 2022, 8, 357. https://doi.org/10.3390/gels8060357
Liu X, Zhang M, Song W, Zhang Y, Yu D-G, Liu Y. Electrospun Core (HPMC–Acetaminophen)–Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery. Gels. 2022; 8(6):357. https://doi.org/10.3390/gels8060357
Chicago/Turabian StyleLiu, Xinkuan, Mingxin Zhang, Wenliang Song, Yu Zhang, Deng-Guang Yu, and Yanbo Liu. 2022. "Electrospun Core (HPMC–Acetaminophen)–Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery" Gels 8, no. 6: 357. https://doi.org/10.3390/gels8060357
APA StyleLiu, X., Zhang, M., Song, W., Zhang, Y., Yu, D. -G., & Liu, Y. (2022). Electrospun Core (HPMC–Acetaminophen)–Shell (PVP–Sucralose) Nanohybrids for Rapid Drug Delivery. Gels, 8(6), 357. https://doi.org/10.3390/gels8060357