Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds
Abstract
:1. Introduction
2. Results and Discussion
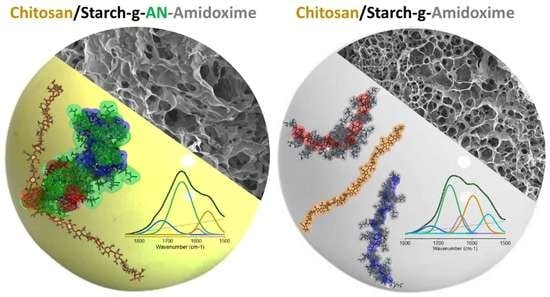
2.1. Starch Functionalization
2.2. Hydrogel Beads of Functionalized Starch and Chitosan
2.3. Swelling Behavior of Hydrogel Beads
2.4. Sorption of Metal Ions by Hydrogel Beads
2.5. Sorption of Dyes
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Starch Functionalization
4.3. Hydrogel Bead Synthesis
4.4. Characterization Methods
4.5. Molecular Dynamics Simulation
4.6. Composite Beads’ Swelling Behavior
4.7. Sorption Experiments
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef] [PubMed]
- Wojnárovits, L.; Földváry, C.M.; Takács, E. Radiation-induced grafting of cellulose for adsoption of hazardous water pollutans: A review. Radiat. Phys. Chem. 2010, 79, 848–862. [Google Scholar] [CrossRef]
- Abdel-Aal, S.E.; Gad, Y.H.; Dessouki, A.M. Use of rice straw and radiation-modified maize starch/acrylonitrile in the treatment of wastewater. J. Hazard. Mater. 2006, 129, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, H.; Wang, L.; ul Abdin, Z.; Chen, Y.; Wang, J.; Zhou, W.; Yang, X.; Khan, R.U.; Zhang, H.; et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015, 5, 67459–67474. [Google Scholar] [CrossRef]
- Loghin, D.F.; Dragan, E.S.; Mihai, M. Comparative chemical modification of starches as a function of their origin: Synthesis and analysis. Rev. Roum. Chim. 2019, 64, 915–921. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and physical modifications of starch for renewable polymeric materials. Mater. Today Sustain. 2020, 7, 100028. [Google Scholar] [CrossRef]
- Wang, X.; Huang, L.; Zhang, C.; Deng, Y.; Xie, P.; Liu, L.; Cheng, J. Research advances in chemical modifications of starch for hydrophobicity and its applications: A review. Carbohydr. Polym. 2020, 240, 116292. [Google Scholar] [CrossRef]
- Ma, X.; Liu, X.; Anderson, D.P.; Chang, P.R. Modification of porous starch for the adsorption of heavy metal ions from aqueous solution. Food Chem. 2015, 181, 133–139. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Boddu, V.M.; Abburi, K.; Talbott, J.L.; Smith, E.D.; Haasch, R. Removal of arsenic (III) and arsenic (V) from aqueous medium using chitosan-coated biosorbent. Water Res. 2008, 42, 633–642. [Google Scholar] [CrossRef]
- Cocarta, A.I.; Gutanu, V.; Dragan, E.S. Comparative sorption of Co2+, Ni2+ and Cr3+ onto chitosan/poly(vinyl amine) composite beads. Cellul. Chem. Technol. 2019, 49, 775–782. [Google Scholar]
- Brion-Roby, R.; Gagnon, J.; Nosrati, S.; Deschênes, J.-S.; Chabot, B. Adsorption and desorption of molybdenum(VI) in contaminated water using a chitosan sorbent. J. Water Process. Eng. 2018, 23, 13–19. [Google Scholar] [CrossRef]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Negm, N.A.; Hefni, H.H.H.; Abd-Elaal, A.A.A.; Badra, E.A.; Abou Kana, M.T.H. Advancement on modification of chitosan biopolymer and its potential applications. Int. J. Biol. Macromol. 2020, 152, 681–702. [Google Scholar] [CrossRef]
- Ali, N.; Khan, A.; Malik, S.; Badshah, S.; Bilal, M.; Iqbal, H.M.N. Chitosan-based green sorbent material for cations removal from an aqueous environment. J. Environ. Chem. Eng. 2020, 8, 104064. [Google Scholar] [CrossRef]
- Apopei, D.F.; Dinu, M.V.; Trochimczuk, A.W.; Dragan, E.S. Sorption isotherms of heavy metal ions onto semi-interpenetrating polymer network cryogels based on polyacrylamide and anionically modified potato starch. Ind. Eng. Chem. Res. 2012, 51, 10462–10471. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Fan, W.; Yi, X.; Wang, Z.; Gao, F.; Li, Y.; Gu, H. Dithiocarbamate-modified starch derivatives with high heavy metal adsorption performance. Carbohydr. Polym. 2016, 136, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Liu, R.; Chen, M.; Li, Z.; Qin, T.; Qian, Y.; Zhao, S.; Liu, M.; Zeng, Q.; Shen, J. Removal of copper ions from water using polysaccharide-constructed hydrogels. Carbohydr. Polym. 2019, 209, 101–110. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Cellulose based grafted biosorbents—Journey from lignocellulose biomass to toxic metal ions sorption applications—A review. J. Mol. Liq. 2017, 232, 62–93. [Google Scholar] [CrossRef]
- Wang, S.; Vincent, T.; Faur, C.; Guibal, E. Modeling competitive sorption of lead and copper ions onto alginate and greenly prepared algal-based beads. Bioresour. Technol. 2017, 231, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Joly, N.; Ghemati, D.; Aliouche, D.; Martin, P. Interaction of metal ions with mono- and polysaccharides for wastewater treatment: A review. Nat. Prod. Chem. Res. 2020, 8, 373. [Google Scholar]
- Chauhan, G.S.; Jaswal, S.C.; Verma, M. Post functionalization of carboxymethylated starch and acrylonitrile based networks through amidoximation for use as ion sorbents. Carbohydr. Polym. 2006, 66, 435–443. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Pourjavadi, A.; Salehi-Rad, M. Modified CMC. 2. Novel carboxymethylcellulose-based poly(amidoxime) chelating resin with high metal sorption capacity. React. Funct. Polym. 2004, 61, 23–31. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Divya, L.; Bringle, C.D.; Suchithra, P.S. Removal of Copper(II) and Zinc(II) from aqueous solutions using a lignocellulosic-based polymeric adsorbent containing amidoxime chelating functional groups. Sep. Sci. Technol. 2010, 45, 2383–2393. [Google Scholar] [CrossRef]
- Liu, X.; Chen, H.; Wang, C.; Qu, R.; Ji, C.; Sun, C.; Zhang, Y. Synthesis of porous acrylonitrile/methyl acrylate copolymer beads by suspended emulsion polymerization and their adsorption properties after amidoximation. J. Hazard. Mater. 2010, 175, 1014–1021. [Google Scholar] [CrossRef]
- Lutfor, M.R.; Silong, S.; Zin, W.M.; Ab Rahman, M.Z.; Ahmad, M.; Haron, J. Preparation and characterization of poly(amidoxime) chelating resin from polyacrylonitrile grafted sago starch. Eur. Polym. J. 2000, 36, 2105–2113. [Google Scholar] [CrossRef]
- Masoumi, A.; Ghaemy, M. Removal of metal ions from water using nanohydrogel tragacanthgum-g-polyamidoxime: Isotherm and kinetic study. Carbohydr. Polym. 2014, 108, 206–215. [Google Scholar] [CrossRef]
- Xu, C.; Wang, J.; Yang, T.; Chen, X.; Liu, X.; Ding, X. Adsorption of uranium by amidoximated chitosan-grafted polyacrylonitrile, using response surface methodology. Carbohydr. Polym. 2015, 121, 79–85. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; El-Bindary, A.A.; Kouta, E.Y.; Guibal, E. Functionalization of polyacrylonitrile/Na-Y-zeolite composite with amidoxime groups for the sorption of Cu(II), Cd(II) and Pb(II) metal ions. Chem. Eng. J. 2018, 332, 727–736. [Google Scholar] [CrossRef]
- Xu, S.; Jin, Y.; Li, R.; Shan, M.; Zhang, Y. Amidoxime modified polymers of intrinsic microporosity/alginate composite hydrogel beads for efficient adsorption of cationic dyes from aqueous solution. J. Colloid Interface Sci. 2022, 607, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Egawa, H.; Kabay, N.; Shuto, T.; Jyo, A. Recovery of uranium from seawater. XII. Preparation and characterization of lightly crosslinked highly porous chelating resins containing amidoxime groups. J. Appl. Polym. Sci. 1992, 46, 129–142. [Google Scholar] [CrossRef]
- Kubota, H.; Shigehisa, Y. Introduction of amidoxime groups into cellulose and its ability to adsorb metal ions. J. Appl. Polym. Sci. 1995, 56, 147–151. [Google Scholar] [CrossRef]
- Meimoun, J.; Wiatz, V.; Saint-Loup, R.; Parcq, J.; Favrelle, A.; Bonnet, F.; Zinck, P. Modification of starch by graft copolymerization. Starch/Stärke 2017, 69, 1600351. [Google Scholar] [CrossRef]
- Rahman, M.L.; Sarjadi, M.S.; Guerin, S.; Sarkar, S.M. Poly (amidoxime) resins for efficient and eco-friendly metal extraction. ACS Appl. Polym. Mater. 2022, 4, 2216–2232. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei, D.F. Synthesis and swelling behavior of pH-sensitive semi-interpenetrating polymer network composite hydrogels based on native and modified potatoes starch as potential sorbent for cationic dyes. Chem. Eng. J. 2011, 178, 252–263. [Google Scholar] [CrossRef]
- Dragan, E.S.; Loghin Apopei, D.F.; Cocarta, A.I. Efficient sorption of Cu2+ by composite chelating sorbents based on potato starch-graft-polyamidoxime embedded in chitosan beads. ACS Appl. Mater. Interfaces 2014, 6, 16577–16592. [Google Scholar] [CrossRef]
- Imberty, A.; Chanzy, H.; Pérez, S.; Bulèon, A.; Tran, V. The double-helical nature of the crystalline part of A-starch. J. Mol. Biol. 1988, 201, 365–378. [Google Scholar] [CrossRef]
- Manek, R.V.; Builders, P.F.; Kolling, W.M.; Emeje, M.; Kunle, O.O. Physicochemical and binder properties of starch obtained from Cyperus esculentus. AAPS PharmSciTech 2012, 13, 379–388. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lim, S.-T. Preparation of nano-sized starch particles by complex formation with n-butanol. Carbohydr. Polym. 2009, 76, 110–116. [Google Scholar] [CrossRef]
- Khatami, M.H.; Barbera, W.; De Haan, H.W. Using geometric criteria to study helix-like structures produced in molecular dynamics simulations of single amylose chains in water. RSC Adv. 2021, 11, 11992–12002. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, M.-M.; Bucatariu, F.; Doroftei, F.; Loghin, D.-F.; Vasiliu, A.-L.; Mihai, M. Multifunctional CaCO3/polyelectrolyte sorbents for heavy metal ions decontamination of synthetic waters. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126084. [Google Scholar] [CrossRef]
- Bucatariu, F.; Ghiorghita, C.A.; Zaharia, M.M.; Schwarz, S.; Simon, F.; Mihai, M. Removal and separation of heavy metal ions from multicomponent simulated waters using silica/polyethyleneimine composite microparticles. ACS Appl. Mater. Interfaces 2020, 12, 37585–37596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, F.; Mu, Y.; Zeng, E.Y.; Meng, Y.; Zhao, X.; Giesy, J.P.; Feng, C.; Wang, P.; Liao, H.; et al. Directly Predicting water quality criteria from physicochemical properties of transition metals. Sci. Rep. 2016, 6, 22515. [Google Scholar] [CrossRef]
- Feizi, M.; Jalali, M. Removal of heavy metals from aqueous solutions using sunflower, potato, canola and walnut shell residues. J. Taiwan Inst. Chem. Eng. 2015, 54, 125–136. [Google Scholar] [CrossRef]
- Arshadi, M.; Amiri, M.J.; Mousavi, S. Kinetic, equilibrium and thermodynamic investigations of Ni(II), Cd(II), Cu(II) and Co(II) adsorption on barley straw ash. Water Resour. Ind. 2014, 6, 1–17. [Google Scholar] [CrossRef]
- Rulıíšek, L.; Vondrášek, J. Coordination geometries of selected transition metal ions (Co2+, Ni2+, Cu2+, Zn2+, Cd2+, and Hg2+) in metalloproteins. J. Inorg. Biochem. 1998, 71, 115–127. [Google Scholar] [CrossRef]
- Chen, Z.; Song, X.; Soh, W.W.M.; Wen, Y.; Zhu, J.; Zhang, M.; Li, J. In situ synthesis of magnetic poly(DMAEAB-co-NIPAm)@Fe3O4 composite hydrogel for removal of dye from water. Gels 2021, 7, 201. [Google Scholar] [CrossRef]
- Santos, C.; Seabra, P.; Veleirinho, B.; Delgadillo, I.; Lopes da Silva, J.A. Acetylation and molecular mass effects on barrier and mechanical properties of shortfin squid chitosan membranes. Eur. Polym. J. 2006, 42, 3277–3285. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Case, D.A.; Ben-Shalom, I.Y.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.I.; Cruzeiro, V.W.D.; Darden, T.A.; Duke, R.E.; Ghoreishi, D.; Gilson, M.K.; et al. AMBER 2018; University of California: San Francisco, CA, USA, 2018. [Google Scholar]
- Dupradeau, F.Y.; Pigache, A.; Zaffran, T.; Savineau, C.; Lelong, R.; Grivel, N.; Lelong, D.; Rosanski, W.; Cieplak, P. The R.E.D. tools: Advances in RESP and ESP charge derivation and force field library building. Phys. Chem. Chem. Phys. 2010, 12, 7821–7839. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahlad, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]










| Sample | Area, µm2 | Perimeter, µm | Feret Diameter, µm | Aspect Ratio |
|---|---|---|---|---|
| CS/PSgAN | 25.26 ± 29.71 | 15.69 ± 8.87 | 5.59 ± 3.21 | 1.35 ± 1.67 |
| CS/WSgAN | 29.50 ± 26.47 | 18.83 ± 11.00 | 6.18 ± 3.98 | 1.80 ± 1.25 |
| CS/RSgAN | 14.03 ± 9.16 | 12.74 ± 4.07 | 4.46 ± 1.48 | 1.15 ± 1.46 |
| CS/PSgAN-Ax | 27.74 ± 34.35 | 16.89 ± 8.69 | 6.17 ± 3.31 | 1.42 ± 1.53 |
| CS/WSgAN-Ax | 34.35 ± 32.53 | 19.27 ± 12.53 | 6.85 ± 4.32 | 1.95 ± 1.62 |
| CS/RSgAN-Ax | 16.89 ± 9.67 | 14.14 ± 4.02 | 5.00 ± 1.43 | 1.26 ± 1.56 |
| CS/PSgAx | 16.28 ± 13.63 | 11.93 ± 5.03 | 4.32 ± 1.70 | 1.29 ± 1.18 |
| CS/WSgAx | 14.37 ± 7.13 | 11.40 ± 3.04 | 3.97 ± 1.31 | 1.31 ± 1.12 |
| CS/RSgAx | 15.52 ± 10.99 | 11.24 ± 4.48 | 4.03 ± 1.62 | 1.58 ± 1.46 |
| Sample | C/N | C/O | ||||
|---|---|---|---|---|---|---|
| PS | WS | RS | PS | WS | RS | |
| Calculated | ||||||
| CS/SgAN | 5.06 | 1.41 | ||||
| CS/SgAN-Ax | 3.54 | 1.24 | ||||
| At surface/In section | ||||||
| CS/SgAN | 7.44/5.46 | 6.56/6.27 | 6.28/6.35 | 2.03/1.95 | 1.89/1.80 | 2.16/1.93 |
| CS/SgAN-Ax | 6.40/4.74 | 6.31/5.35 | 5.80/5.31 | 2.17/2.05 | 1.85/1.70 | 1.85/1.79 |
| CS/SgAx | 6.43/4.91 | 6.21/5.46 | 5.45/5.65 | 1.89/1.81 | 1.87/1.89 | 1.84/1.67 |
| System | Number of Water Molecules TIP3P | Number of Starch/CS Molecules | Length of the Grafted Chain | Box Size | Simulation Time (ns) |
|---|---|---|---|---|---|
| Sg | 10,914 | 2/- | - | 70 × 70 × 70 | 200 |
| SgAN | 16,988 | 2/- | 3 | 85 × 85 × 85 | 200 |
| SgAx | 16,852 | 2/- | 3 | 81 × 81 × 81 | 200 |
| SgAN + CS | 72,345 | 2/1 | 3 | 130 × 130 × 130 | 200 |
| SgAx + CS | 72,389 | 2/1 | 3 | 130 × 130 × 130 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loghin, D.F.; Bazarghideanu, M.M.; Vasiliu, S.; Racovita, S.; Zaharia, M.-M.; Vasiliu, T.; Mihai, M. Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds. Gels 2022, 8, 549. https://doi.org/10.3390/gels8090549
Loghin DF, Bazarghideanu MM, Vasiliu S, Racovita S, Zaharia M-M, Vasiliu T, Mihai M. Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds. Gels. 2022; 8(9):549. https://doi.org/10.3390/gels8090549
Chicago/Turabian StyleLoghin, Diana Felicia, Melinda Maria Bazarghideanu, Silvia Vasiliu, Stefania Racovita, Marius-Mihai Zaharia, Tudor Vasiliu, and Marcela Mihai. 2022. "Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds" Gels 8, no. 9: 549. https://doi.org/10.3390/gels8090549
APA StyleLoghin, D. F., Bazarghideanu, M. M., Vasiliu, S., Racovita, S., Zaharia, M. -M., Vasiliu, T., & Mihai, M. (2022). Hydrogel Beads of Amidoximated Starch and Chitosan as Efficient Sorbents for Inorganic and Organic Compounds. Gels, 8(9), 549. https://doi.org/10.3390/gels8090549