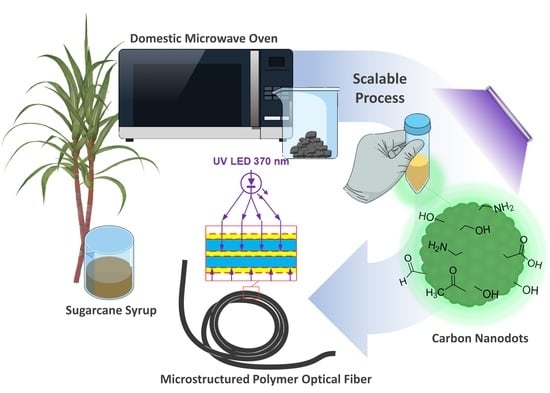
Synthesis of Carbon Nanodots from Sugarcane Syrup, and Their Incorporation into a Hydrogel-Based Composite to Fabricate Innovative Fluorescent Microstructured Polymer Optical Fibers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Carbon Nanodots (CNDs) Synthesis and Characterization
2.2. Preparation of Hydrogel–CNDs Fluorescent Composite
2.3. Photophysical Properties of CNDs and Hydrogel-CNDs Composite
2.4. Fabrication of Microstructured Polymer Optical Fibers (mPOF) Using the Hydrogel–CNDs Composite
3. Conclusions
4. Experimental Section
4.1. Materials and Methods
4.2. Synthesis of CNDs from Sugar Cane Syrup
4.3. Purification of the CNDs
4.4. Preparation of Hydrogel–CNDs Composite
4.5. Preparation of Microstructured Polymer Optical Fiber (mPOF) with Hydrogel–CNDs Composite
4.6. Fourier Transform Infrared Spectroscopy (FTIR)
4.7. Dynamic Light Scattering (DLS)
4.8. Atomic Force Microscopy (AFM)
4.9. Absorption Spectroscopy in the UV-Vis
4.10. Emission Spectroscopy
4.11. Refractive Index
4.12. Quantum Yield
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Hu, A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 4, 11582–11603. [Google Scholar] [CrossRef]
- Ðorđević, L.; Arcudi, F.; D’Urso, A.; Cacioppo, M.; Micali, N.; Bürgi, T.; Purrello, R.; Prato, M. Design principles of chiral carbon nanodots help convey chirality from molecular to nanoscale level. Nat. Commun. 2018, 9, 3442. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Zhu, L.; Choi, K.Y.; Guo, N.; Guo, J.; Tackett, K.; Anilkumar, P.; Liu, G.; Quan, Q.; et al. Effect of Injection Routes on the Biodistribution, Clearance, and Tumor Uptake of Carbon Dots. ACS Nano 2013, 7, 5684–5693. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic applications. Nano Today 2016, 11, 565–586. [Google Scholar] [CrossRef]
- Tian, X.-T.; Yin, X.-B. Carbon Dots, Unconventional Preparation Strategies, and Applications Beyond Photoluminescence. Small 2019, 15, 1901803. [Google Scholar] [CrossRef]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef]
- Cadranel, A.; Margraf, J.T.; Strauss, V.; Clark, T.; Guldi, D.M. Carbon Nanodots for Charge-Transfer Processes. Acc. Chem. Res. 2019, 52, 955–963. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qu, K.; Xu, B.; Ren, J.; Qu, X. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J. Mater. Chem. 2011, 21, 2445–2450. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Synthesis, Separation, and Characterization of Small and Highly Fluorescent Nitrogen-Doped Carbon NanoDots. Angew. Chem. Int. Ed. 2016, 55, 2107–2112. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Weng, P.; Tang, Z.; Guo, B. Sustainable Carbon Nanodots with Tunable Radical Scavenging Activity for Elastomers. ACS Sustain. Chem. Eng. 2016, 4, 247–254. [Google Scholar]
- Duarah, R.; Karak, N. Facile and Ultrafast Green Approach to Synthesize Biobased Luminescent Reduced Carbon Nanodot: An Efficient Photocatalyst. ACS Sustain. Chem. Eng. 2017, 5, 9454–9466. [Google Scholar] [CrossRef]
- Devi, R.; Dhamodharan, R. Sustainable Process for Separating Chitin and Simultaneous Synthesis of Carbon Nanodots from Shellfish Waste Using 2% Aqueous Urea Solution. ACS Sustain. Chem. Eng. 2018, 6, 11313–11325. [Google Scholar]
- Ran, X.; Qu, Q.; Li, L.; Zuo, L.; Zhang, S.; Gui, J.; Kang, Y.; Yang, L. One-Step Synthesis of Novel Photoluminescent Nitrogen-Rich Carbon Nanodots from Allylamine for Highly Sensitive and Selective Fluorescence Detection of Trinitrophenol and Fluorescent Ink. ACS Sustain. Chem. Eng. 2018, 6, 11716–11723. [Google Scholar] [CrossRef]
- Ravishankar, K.; Shelly, K.M.; Narayanan, A.; Dhamodharan, R. Rapid, Solvent-Free Synthesis of Amorphous, Photoluminescent, Carbon Nanodots from Imidazole and Maleic Anhydride Solids. ACS Sustain. Chem. Eng. 2019, 7, 13206–13216. [Google Scholar]
- Zhang, Z.; Sun, W.; Wu, P. Highly Photoluminescent Carbon Dots Derived from Egg White: Facile and Green Synthesis, Photoluminescence Properties, and Multiple Applications. ACS Sustain. Chem. Eng. 2015, 3, 1412–1418. [Google Scholar] [CrossRef]
- Sharma, V.; Tiwari, P.; Mobin, S.M. Sustainable carbon-dots: Recent advances in green carbon dots for sensing and bioimaging. J. Mater. Chem. B 2017, 5, 8904–8924. [Google Scholar] [CrossRef]
- Xu, H.; Xie, L.; Hakkarainen, M. Coffee-Ground-Derived Quantum Dots for Aqueous Processable Nanoporous Graphene Membranes. ACS Sustain. Chem. Eng. 2017, 5, 5360–5367. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-Derived Carbon Dots and Their Applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar]
- Surendran, P.; Lakshmanan, A.; Vinitha, G.; Gopal, R.; Rameshkumar, P. Facile preparation of high fluorescent carbon quantum dots from orange waste peels for nonlinear optical applications. Luminescence 2019, 35, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Luo, Z.; Lu, Y.; Somers, L.A.; Johnson, A.T.C. High Yield Preparation of Macroscopic Graphene Oxide Membranes. J. Am. Chem. Soc. 2009, 131, 898–899. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef]
- Zuo, P.; Lu, X.; Sun, Z.; Guo, Y.; He, H. A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots. Microchim. Acta 2016, 183, 519–542. [Google Scholar] [CrossRef]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for excitation-dependent photoluminescence from graphene quantum dots and other graphene oxide derivates: Consensus, debates and challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef]
- Li, H.; He, X.; Kang, Z.; Huang, H.; Liu, Y.; Liu, J.; Lian, S.; Tsang, C.H.A.; Yang, X.; Lee, S.-T. Water-Soluble Fluorescent Carbon Quantum Dots and Photocatalyst Design. Angew. Chem. Int. Ed. 2010, 49, 4430–4434. [Google Scholar] [CrossRef]
- Gupta, V.; Chaudhary, N.; Srivastava, R.; Sharma, G.D.; Bhardwaj, R.; Chand, S. Luminscent Graphene Quantum Dots for Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 9960–9963. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Du, C.; Zhuang, Z.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar]
- Tian, Z.; Zhang, X.; Li, D.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Zboril, R.; Rogach, A.L. Full-Color Inorganic Carbon Dot Phosphors for White-Light-Emitting Diodes. Adv. Optical Mater. 2017, 5, 1700416. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Rationally Designed Carbon Nanodots towards Pure White-Light Emission. Angew. Chem. Int. Ed. 2017, 56, 4170–4173. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Guo, B.; Liu, F.; Zeng, Q.; Yu, C.; Du, X.; Jin, G.; Feng, T.; Zhu, S.; Li, F.; et al. Cathode and Anode Interlayers Based on Polymer Carbon Dots via Work Function Regulation for Efficient Polymer Solar Cells. Adv. Mater. Interfaces 2018, 5, 1701519. [Google Scholar] [CrossRef]
- Li, H.-J.; Sun, X.; Xue, F.; Ou, N.; Sun, B.-W.; Qian, D.-J.; Chen, M.; Wang, D.; Yang, J.; Wang, X. Redox Induced Fluorescence On–Off Switching Based on Nitrogen Enriched Graphene Quantum Dots for Formaldehyde Detection and Bioimaging. ACS Sustain. Chem. Eng. 2018, 6, 1708–1716. [Google Scholar] [CrossRef]
- Arcudi, F.; Đorđević, L.; Prato, M. Design, Synthesis, and Functionalization Strategies of Tailored Carbon Nanodots. Acc. Chem. Res. 2019, 52, 2070–2079. [Google Scholar] [CrossRef]
- Chen, B.; Xie, H.; Wang, S.; Guo, Z.; Hu, Y.; Xie, H. UV light-tunable fluorescent inks and polymer hydrogel films based on carbon nanodots and lanthanide for enhancing anti-counterfeiting. Luminescence 2019, 34, 437–443. [Google Scholar] [CrossRef]
- Gonçalves, H.M.R.; Duarte, A.J.; Esteves da Silva, J.C.G. Optical fiber sensor for Hg(II) based on carbon dots. Biosens. Bioelectron. 2010, 26, 1302–1306. [Google Scholar] [CrossRef]
- Lin, H.; Huang, J.; Ding, L. A Recyclable Optical Fiber Sensor Based on Fluorescent Carbon Dots for the Determination of Ferric Ion Concentrations. J. Lightwave Technol. 2019, 37, 4815–4822. [Google Scholar] [CrossRef]
- Yap, S.H.K.; Chan, K.K.; Zhang, G.; Tjin, S.C.; Yong, K.-T. Carbon Dot-functionalized Interferometric Optical Fiber Sensor for Detection of Ferric Ions in Biological Samples. ACS Appl. Mater. Interf. 2019, 11, 28546–28553. [Google Scholar] [CrossRef] [PubMed]
- Borges, E.; Lopes, M.; Amorim, H. Impact of sugar cane juice chemical composition on clarification and VHP sugar quality. Int. Sugar J. 2012, 114, 552–558. [Google Scholar]
- Soares, M.C.P.; Perli, G.; Bartoli, J.R.; Bertuzzi, D.L.; Taketa, T.B.; Bataglioli, R.A.; Suzuki, C.K.; Ornelas, C.; Fujiwara, E. Fast Microwave-Assisted Synthesis of Green-Fluorescent Carbon Nanodots from Sugarcane Syrup. In Proceedings of the 2019 SBFoton International Optics and Photonics Conference (SBFoton IOPC), Sao Paulo, Brazil, 7–9 October 2019. [Google Scholar]
- Ornelas, C.; Ruiz, J.; Belin, C.; Astruc, D. Giant Dendritic Molecular Electrochrome Batteries with Ferrocenyl and Pentamethylferrocenyl Termini. J. Am. Chem. Soc. 2009, 131, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Astruc, D.; Ornelas, C.; Ruiz, J. Dendritic Molecular Electrochromic Batteries Based on Redox-Robust Metallocenes. Chem. Eur. J. 2009, 15, 8936–8944. [Google Scholar] [CrossRef]
- Han, Y.; Lv, S. Synthesis of chemically crosslinked pullulan/gelatin-based extracellular matrix-mimetic gels. Int. J. Biolog. Macromol. 2019, 122, 1262–1270. [Google Scholar] [CrossRef]
- Burey, P.; Bhandari, B.R.; Howes, T.; Gidley, M.J. Hydrocolloid Gel Particles: Formation, Characterization, and Application. Crit. Rev. Food Sci. Nutr. 2008, 48, 361–377. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Yang, C. Ratiometric fluorescence sensor for Fe3+ ions detection based on quantum dot-doped hydrogel optical fiber. Sens. Actuators B Chem. 2018, 264, 52–58. [Google Scholar] [CrossRef]
- Schyrr, B.; Boder-Pasche, S.; Ischer, R.; Smajda, R.; Voirin, G. Fiber-optic protease sensor based on the degradation of thin gelatin films. Sens. Bio-Sens. Res. 2015, 3, 65–73. [Google Scholar] [CrossRef]
- Rahimi, M.; Mahani, M.; Hassani, Z. Carbon quantum dots fluorescence quenching for potassium optode construction. Luminescence 2019, 34, 402–406. [Google Scholar] [CrossRef]
- Choi, M.; Choi, J.W.; Kim, S.; Nizamoglu, S.; Hahn, S.K.; Yun, S.H. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat. Photonics 2013, 7, 987–994. [Google Scholar] [CrossRef]
- Choi, M.; Humar, M.; Kim, S.; Yun, S.-H. Step-Index Optical Fiber Made of Biocompatible Hydrogels. Adv. Mater. 2015, 27, 4081–4086. [Google Scholar] [CrossRef] [PubMed]
- Djabourov, M. Gelation—A review. Polym. Inter. 1991, 25, 135–143. [Google Scholar] [CrossRef]
- Bohidar, H.B.; Jena, S.S. Kinetics of sol–gel transition in thermoreversible gelation of gelatin. J. Chem. Phys. 1993, 98, 8970–8977. [Google Scholar] [CrossRef]
- Finer, E.G.; Franks, F.; Phillips, M.C.; Suggett, A. Gel formation from solutions of single-chain gelatin. Biopolymers 1975, 14, 1995–2005. [Google Scholar] [CrossRef]
- Beadie, G.; Brindza, M.; Flynn, R.A.; Rosenberg, A.; Shirk, J.S. Refractive index measurements of poly(methyl methacrylate) (PMMA) from 0.4 to 1.6 µm. Appl. Opt. 2015, 54, F139–F143. [Google Scholar] [CrossRef]
- Suppan, P. Invited review solvatochromic shifts: The influence of the medium on the energy of electronic states. J. Photochem. Photobiol. A Chem. 1990, 50, 293–330. [Google Scholar] [CrossRef]
- Eijkelenborg, M.A.; Large, M.C.J.; Argyros, A.; Zagari, J.; Manos, S.; Issa, N.A.; Bassett, I.; Fleming, S.; McPhedran, R.C.; Sterke, C.M.; et al. Microstructured polymer optical fibre. Opt. Express 2001, 9, 319–327. [Google Scholar] [CrossRef]
- Yang, X.; Yu, W.; Liu, Z.; Yang, J.; Zhang, Y.; Kong, D.; Long, Q.; Yuan, T.; Cao, J.; Yuan, L.; et al. Optofluidic twin-core hollow fiber interferometer for label-free sensing of the streptavidin-biotin binding. Sens. Actuators B Chem. 2018, 277, 353–359. [Google Scholar] [CrossRef]
- Limpert, J.; Schreiber, T.; Nolte, S.; Zellmer, H.; Tünnermann, A.; Iliew, R.; Lederer, F.; Broeng, J.; Vienne, G.; Petersson, A.; et al. High-power air-clad large-mode-area photonic crystal fiber laser. Opt. Express 2003, 11, 818–823. [Google Scholar] [CrossRef]
- Zenteno, L. High-power double-clad fiber lasers. J. Lightwave Technol. 1993, 11, 1435–1446. [Google Scholar] [CrossRef]
- Chen, Z.J.; Grudinin, A.B.; Porta, J.; Minelly, J.D. Enhanced Q switching in double-clad fiber lasers. Opt. Lett. 1998, 23, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Hideur, A.; Chartier, T.; Özkul, C.; Sanchez, F. Dynamics and stabilization of a high power side-pumped Yb-doped double-clad fiber laser. Opt. Commun. 2000, 186, 311–317. [Google Scholar] [CrossRef]
- Wang, Y.; Po, H. Dynamic Characteristics of Double-Clad Fiber Amplifiers for High-Power Pulse Amplification. J. Lightwave Technol. 2003, 21, 2262–2270. [Google Scholar] [CrossRef]
- Limpert, J.; Deguil-Robin, N.; Manek-Hönninger, I.; Salin, F.; Röser, F.; Liem, A.; Schreiber, T.; Nolte, S.; Zellmer, H.; Tünnermann, A.; et al. High-power rod-type photonic crystal fiber laser. Opt. Express 2005, 13, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Yan, P.; Huang, L.; Liu, Q.; Gong, M. Stimulated Brillouin scattering phenomena in a nanosecond linearly polarized Yb-doped double-clad fiber amplifier. Laser Phys. Lett. 2007, 4, 376–381. [Google Scholar] [CrossRef]
- Filippov, V.; Chamorovskii, Y.; Kerttula, J.; Golant, K.; Pessa, M.; Okhotnikov, O.G. Double clad tapered fiber for high power applications. Opt. Express 2008, 16, 1929–1944. [Google Scholar] [CrossRef]
- Filippov, V.; Kerttula, J.; Chamorovskii, Y.; Golant, K.; Okhotnikov, O.G. Highly efficient 750 W tapered double-clad ytterbium fiber laser. Opt. Express 2010, 18, 12499–12512. [Google Scholar] [CrossRef]
- Doya, V.; Legrand, O.; Mortessagne, F. Optimized absorption in a chaotic double-clad fiber amplifier. Opt. Lett. 2001, 26, 872–874. [Google Scholar] [CrossRef]
- Amrani, F.; Haboucha, A.; Salhi, M.; Leblond, H.; Komarov, A.; Grelu, P.; Sanchez, F. Passively mode-locked erbium-doped double-clad fiber laser operating at the 322nd harmonic. Opt. Lett. 2009, 34, 2120–2122. [Google Scholar] [CrossRef]
- Abedin, K.S.; Fini, J.M.; Thierry, T.F.; Zhu, B.; Yan, M.F.; Bansal, L.; Dimarcello, F.V.; Monberg, E.M.; DiGiovanni, D.J. Seven-core erbium-doped double-clad fiber amplifier pumped simultaneously by side-coupled multimode fiber. Opt. Lett. 2014, 39, 993–996. [Google Scholar] [CrossRef]
- Liu, C.; Ye, C.; Luo, Z.; Cheng, H.; Wu, D.; Zheng, Y.; Liu, Z.; Qu, B. High-energy passively Q-switched 2 μm Tm3+-doped double-clad fiber laser using graphene-oxide-deposited fiber taper. Opt. Express 2013, 21, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Liu, C.; Huang, Y.; Wu, D.; Wu, J.; Xu, H.; Cai, Z.; Lin, Z.; Sun, L.; Weng, J. Topological-Insulator Passively Q-Switched Double-Clad Fiber Laser at 2 μm Wavelength. IEEE J. Selec. Top. Quantum Electron. 2014, 20, 1–8. [Google Scholar]
- Laroche, M.; Chardon, A.M.; Nilsson, J.; Shepherd, D.P.; Clarkson, W.A.; Girard, S.; Moncorgé, R. Compact diode-pumped passively Q-switched tunable Er–Yb double-clad fiber laser. Opt. Lett. 2002, 27, 1980–1982. [Google Scholar] [CrossRef]
- Sobon, G.; Krzempek, K.; Kaczmarek, P.; Abramski, K.M.; Nikodem, M. 10GHz passive harmonic mode-locking in Er–Yb double-clad fiber laser. Opt. Commun. 2011, 284, 4203–4206. [Google Scholar] [CrossRef]
- Yelin, D.; Bouma, B.E.; Yun, S.H.; Tearney, G.J. Double-clad fiber for endoscopy. Opt. Lett. 2004, 29, 2408–2410. [Google Scholar] [CrossRef]
- Wang, L.; Choi, H.Y.; Jung, Y.; Lee, B.H.; Kim, K.-T. Optical probe based on double-clad optical fiber for fluorescence spectroscopy. Opt. Express 2007, 15, 17681–17689. [Google Scholar] [CrossRef]
- Lemire-Renaud, S.; Rivard, M.; Strupler, M.; Morneau, D.; Verpillat, F.; Daxhelet, X.; Godbout, N.; Boudoux, C. Double-clad fiber coupler for endoscopy. Opt. Express 2010, 18, 9755–9764. [Google Scholar] [CrossRef]
- Liang, S.; Sun, C.; Saidi, A.; Jing, J.; Liu, G.; Li, J.; Zhang, J.; Chen, Z.; Narula, J. Intravascular atherosclerotic imaging with combined fluorescence and optical coherence tomography probe based on a double-clad fiber combiner. J. Biomed. Opt. 2012, 17, 070501. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Mejía, F.; Cordeiro, C.M.B.; Andrés, P.; Silvestre, E. Broadband dispersion compensation using inner cladding modes in photonic crystal fibers. Opt. Express 2012, 20, 3467–3472. [Google Scholar] [CrossRef]
- Wiederhecker, G.S.; Cordeiro, C.M.B.; Couny, F.; Benabid, F.; Maier, S.A.; Knight, J.C.; Cruz, C.H.B.; Fragnito, H.L. Field enhancement within an optical fibre with a subwavelength air core. Nat. Photon 2007, 1, 115–118. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Li, H.; Deng, Y.; Xi, L.; Tang, X.; Zhang, W. The Orbital Angular Momentum Modes Supporting Fibers Based on the Photonic Crystal Fiber Structure. Crystals 2017, 7, 286. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perli, G.; Soares, M.C.P.; Cabral, T.D.; Bertuzzi, D.L.; Bartoli, J.R.; Livi, S.; Duchet-Rumeau, J.; Cordeiro, C.M.B.; Fujiwara, E.; Ornelas, C. Synthesis of Carbon Nanodots from Sugarcane Syrup, and Their Incorporation into a Hydrogel-Based Composite to Fabricate Innovative Fluorescent Microstructured Polymer Optical Fibers. Gels 2022, 8, 553. https://doi.org/10.3390/gels8090553
Perli G, Soares MCP, Cabral TD, Bertuzzi DL, Bartoli JR, Livi S, Duchet-Rumeau J, Cordeiro CMB, Fujiwara E, Ornelas C. Synthesis of Carbon Nanodots from Sugarcane Syrup, and Their Incorporation into a Hydrogel-Based Composite to Fabricate Innovative Fluorescent Microstructured Polymer Optical Fibers. Gels. 2022; 8(9):553. https://doi.org/10.3390/gels8090553
Chicago/Turabian StylePerli, Gabriel, Marco C. P. Soares, Thiago D. Cabral, Diego L. Bertuzzi, Julio R. Bartoli, Sébastien Livi, Jannick Duchet-Rumeau, Cristiano M. B. Cordeiro, Eric Fujiwara, and Catia Ornelas. 2022. "Synthesis of Carbon Nanodots from Sugarcane Syrup, and Their Incorporation into a Hydrogel-Based Composite to Fabricate Innovative Fluorescent Microstructured Polymer Optical Fibers" Gels 8, no. 9: 553. https://doi.org/10.3390/gels8090553
APA StylePerli, G., Soares, M. C. P., Cabral, T. D., Bertuzzi, D. L., Bartoli, J. R., Livi, S., Duchet-Rumeau, J., Cordeiro, C. M. B., Fujiwara, E., & Ornelas, C. (2022). Synthesis of Carbon Nanodots from Sugarcane Syrup, and Their Incorporation into a Hydrogel-Based Composite to Fabricate Innovative Fluorescent Microstructured Polymer Optical Fibers. Gels, 8(9), 553. https://doi.org/10.3390/gels8090553