Processing of Calcium Magnesium Silicates by the Sol–Gel Route
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gels Characterization
2.2. Ceramics Characterization
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sol–Gel Method
4.3. Materials Characterization
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Ba, Z.; Chen, Z.; Huang, Y.; Feng, D.; Zhao, Q.; Zhu, J.; Wu, D. Nanoporous diopside modulates biocompatibility, degradability and osteogenesis of bioactive scaffolds of gliadin-based composites for new bone formation. Int. J. Nanomed. 2018, 13, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Dasan, A.; Kraxner, J.; Grigolato, L.; Savio, G.; Elsayed, H.; Galusek, D.; Bernardo, E. 3D printing of hierarchically porous lattice structures based on akermanite glass microspheres and reactive silicone binder. J. Funct. Biomater. 2022, 13, 8. [Google Scholar] [CrossRef] [PubMed]
- Hafezi-Ardakani, M.; Moztarzadeh, F.; Rabiee, M.; Talebi, A.R.; Abasi-shahni, M.; Fesahat, F.; Sadeghian, F. Sol-gel synthesis and apatite-formation ability of nanostructure merwinite (Ca3MgSi2O8) as a novel bioceramic. J. Ceram. Proc. Res. 2010, 11, 765–768. [Google Scholar]
- Han, Z.; Gao, C.; Feng, P.; Shen, Y.; Shuai, C.; Peng, S. Silicon carbide whiskers reinforced akermanite scaffolds for tissue engineering. RSC Adv. 2014, 4, 36868–36874. [Google Scholar] [CrossRef]
- Liu, T.; Deng, Y.; Gao, C.; Feng, P.; Shuai, C.; Peng, S. Analysis of 3D printed diopside scaffolds properties for tissue engineering. Mater. Sci. 2015, 21, 590–594. [Google Scholar] [CrossRef]
- Dasan, A.; Elsayed, H.; Kraxner, J.; Galusek, D.; Bernardo, E. Hierarchically porous 3D-printed akermanite scaffolds from silicones and engineered fillers. J. Eur. Ceram. Soc. 2019, 39, 4445–4449. [Google Scholar] [CrossRef]
- Han, Z.; Feng, P.; Gao, C.; Shen, Y.; Shuai, C.; Peng, S. Microstructure, mechanical properties and in vitro bioactivity of akermanite scaffolds fabricated by laser sintering. Biomed. Mater. Eng. 2014, 24, 2073–2080. [Google Scholar] [CrossRef]
- Shahrouzifar, M.R.; Salahinejad, E. Strontium doping into diopside tissue engineering scaffolds. Ceram. Int. 2019, 45, 10176–10181. [Google Scholar] [CrossRef]
- Pang, S.; Wu, D.; Kamutzki, F.; Kurreck, J.; Gurlo, A.; Hanaor, D.A.H. High performing additively manufactured bone scaffolds based on copper substituted diopside. Mater. Des. 2022, 215, 110480. [Google Scholar] [CrossRef]
- Collin, M.S.; Venkatraman, S.K.; Mohana, S.; Sumathi, S.; Drweesh, E.A.; Elnagar, M.M.; Mosa, E.S.; Sasikumar, S. Solution combustion synthesis of functional diopside, akermanite, and merwinite bioceramics: Excellent biomineralization, mechanical strength, and antibacterial ability. Mater. Today Commun. 2021, 27, 102365. [Google Scholar] [CrossRef]
- Ansari, M.; Malmir, F.; Salati, A. Preparation and characterization of akermanite/merwinite scaffolds for bone tissue repair. J. Biomim. Biomater. Biomed. Eng. 2020, 44, 73–81. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vivo biocompatibility of Mg implants surface modified by nanostructured merwinite/PEO. J. Mater. Sci. Mater. Med. 2015, 26, 184. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Y.; Emadi, R.; Kharaziha, M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater. Chem. Phys. 2017, 194, 356–366. [Google Scholar] [CrossRef]
- Dong, X.; Li, H.; Lingling, E.; Cao, J.; Guo, B. Bioceramic akermanite enhanced vascularization and osteogenic differentiation of human induced pluripotent stem cells in 3D scaffolds: In vitro and vivo. RSC Adv. 2019, 9, 25462–25470. [Google Scholar] [CrossRef] [PubMed]
- Arastouei, M.; Khodaei, M.; Atyabi, S.M.; Nodoushan, M.J. Poly lactic acid-akermanite composite scaffolds prepared by fused filament fabrication for bone tissue engineering. J. Mater. Res. Technol. 2020, 9, 14540–14548. [Google Scholar] [CrossRef]
- Birhanu, G.; Doosti-Telgerd, M.; Zandi-Karimi, A.; Karimi, Z.; Daryasari, M.P.; Javar, H.A.; Seyedjafari, E. Enhanced proliferation and osteogenic differentiation of mesenchymal stem cells by diopside coated Poly-L-lactic Acid-Based nanofibrous scaffolds. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 707–716. [Google Scholar] [CrossRef]
- Nadernezhad, A.; Torabinejad, B.; Hafezi, M.; Baghaban-Eslaminejad, M.; Bagheri, F.; Najafi, F. Poly (lactic-co-glycolic)/nanostructured merwinite porous composites for bone tissue engineering: Structural and in vitro characterization. J. Adv. Mater. Proc. 2014, 2, 13–24. [Google Scholar]
- Bafandeh, M.R.; Mojarrabian, H.M.; Doostmohammadi, A. Poly (vinyl alcohol)/chitosan/akermanite nanofibrous scaffolds prepared by electrospinning. J. Macromol. Sci. B Phys. 2019, 58, 749–759. [Google Scholar] [CrossRef]
- Ou, J.; Yin, G.F.; Zhou, D.L.; Chen, X.C.; Yao, Y.D.; Yang, W.Z.; Wu, B.L.; Xue, M.; Cui, J.; Zhu, W.F.; et al. Preparation of merwinite with apatite-forming ability by sol-gel process. Key Eng. Mater. 2007, 330, 67–70. [Google Scholar] [CrossRef]
- Shuai, C.; Han, Z.; Feng, P.; Gao, C.; Xiao, T.; Peng, S. Akermanite scaffolds reinforced with boron nitride nanosheets in bone tissue engineering. J. Mater. Sci. Mater. Med. 2015, 26, 188. [Google Scholar] [CrossRef]
- Nezafati, N.; Hafezi, M.; Zamanian, A.; Yasaei, M.; Mohammadi, M.B. Preparation and characterization of a novel nano-structured merwinite scaffold prepared by freeze casting method. In Proceedings of the 5th International Conference on Nanostructures, Kish Island, Iran, 11–13 August 2014; pp. 58–60. [Google Scholar]
- Venkatraman, S.K.; Choudhary, R.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Suresh, A.; Abraham, J.; Swamiappan, S. Biomineralization, mechanical, antibacterial and biological investigation of larnite and rankinite bioceramics. Mater. Sci. Eng. C 2021, 118, 111466. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.K.; Choudhary, R.; Krishnamurithy, G.; Raghavendran, H.R.B.; Murali, M.R.; Kamarul, T.; Suresh, A.; Abraham, J.; Praharaj, S.; Swamiappan, S. Comparative investigation on antibacterial, biological and mechanical behaviour of monticellite and diopside derived from biowaste for bone regeneration. Mater. Chem. Phys. 2022, 286, 126157. [Google Scholar] [CrossRef]
- Reddy, P.M.; Lakshmi, R.; Dass, F.P.; Swamiappan, S. Synthesis, characterization and formulation of sodium calcium silicate bioceramic for drug delivery applications. Sci. Eng. Compos. Mater. 2014, 23, 375–380. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawamura, N.; Nonami, T. Diopside synthesized by sol-gel method as phosphorus adsorption material: Evaluation of apatite deposition in pseudo body solution. Trans. Mater. Res. Soc. Jpn. 2019, 44, 17–23. [Google Scholar] [CrossRef]
- Choudhary, R.; Koppala, S.; Swamiappan, S. Bioactivity studies of calcium magnesium silicate prepared from eggshell waste by sol-gel combustion synthesis. J. Asian Ceram. Soc. 2015, 3, 173–177. [Google Scholar] [CrossRef]
- Schumacher, T.C.; Volkmann, E.; Yilmaz, R.; Wolf, A.; Treccani, L.; Rezwan, K. Mechanical evaluation of calcium-zirconium-silicate (baghdadite) obtained by a direct solid-state synthesis route. J. Mech. Behav. Biomed. Mater. 2014, 34, 294–301. [Google Scholar] [CrossRef]
- Mohammadi, H.; Ismail, Y.M.B.; Shariff, K.A.; Noor, A.F.M. Effect of substitutional strontium on mechanical properties of akermanite ceramic prepared by solid-state sintering. Mater. Today Proc. 2019, 17, 929–936. [Google Scholar] [CrossRef]
- Sharafabadi, A.K.; Abdellahi, M.; Kazemi, A.; Khandan, A.; Ozada, N. A novel and economical route for synthesizing akermanite (Ca2MgSi2O7) nano-bioceramic. Mater. Sci. Eng. C 2017, 71, 1072–1078. [Google Scholar] [CrossRef]
- Iwata, N.Y.; Tsunakawa, S.; Tanaka, M.; Utsu, T.; Matsumoto, K. Improvements of apatite-forming abilities on pure and sodium-containing diopside substrates using porous diopside thin films as nucleating agent. MRS Online Proc. Libr. 1999, 599, 169–174. [Google Scholar] [CrossRef]
- Gheisari Dehsheikh, H.; Karamian, E. Characterization and synthesis of hardystonite (HT) as a novel nanobioceramic powder. Nanomed. J. 2016, 3, 143–146. [Google Scholar]
- Gheisari, H.; Karamian, E.; Soheily, A. Survey and evaluation of merwinite (MW) as a new nanobioceramic powder. J. Nanoanal. 2020, 7, 225–229. [Google Scholar]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.I. Akermanite-based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Hafezi-Ardakani, M.; Moztarzadeh, F.; Rabiee, M.; Talebi, A.R. Synthesis and characterization of nanocrystalline merwinite (Ca3Mg(SiO4)2) via sol-gel method. Ceram. Int. 2011, 37, 175–180. [Google Scholar] [CrossRef]
- Iwata, N.Y.; Lee, G.H.; Tsunakawa, S.; Tokuoka, Y.; Kawashima, N. Preparation of diopside with apatite-forming ability by sol-gel process using metal alkoxide and metal salts. Colloids Surf. B Biointerfaces 2004, 33, 1–6. [Google Scholar] [CrossRef]
- Lombardi, M.; Cacciotti, I.; Bianco, A.; Montanaro, L. RKKP bioactive glass-ceramic material through an aqueous sol-gel process. Ceram. Int. 2015, 41, 3371–3380. [Google Scholar] [CrossRef]
- Voicu, G.; Ene, V.L.; Sava, D.F.; Surdu, V.A.; Busuioc, C. Sol-gel derived vitroceramic materials for biomedical applications. J. Non-Cryst. Solids 2016, 449, 75–82. [Google Scholar] [CrossRef]
- Duman, S.; Bulut, B. Effect of akermanite powders on mechanical properties and bioactivity of chitosan-based scaffolds produced by 3D-bioprinting. Ceram. Int. 2021, 47, 13912–13921. [Google Scholar] [CrossRef]
- Naga, S.M.; El-Maghraby, H.F.; Mahmoud, E.M.; Killinger, A.; Gadow, R. Hydroxyapatite/diopside porous scaffolds: Preparation and in vitro study. Interceram 2019, 68, 22–29. [Google Scholar] [CrossRef]
- Bigham, A.; Hassanzadeh-Tabrizi, S.A.; Khamsehashari, A.; Chami, A. Surfactant-assisted sol–gel synthesis and characterization of hierarchical nanoporous merwinite with controllable drug release. J. Sol-Gel Sci. Technol. 2018, 87, 618–625. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Synthesis and apatite-formation ability of akermanite. Mater. Lett. 2004, 58, 2415–2417. [Google Scholar] [CrossRef]
- No, Y.; Li, J.; Zreiqat, H. Doped calcium silicate ceramics: A new class of candidates for synthetic bone substitutes. Materials 2017, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Nezafati, N.; Hafezi, M.; Zamanian, A.; Naserirad, M. Effect of adding nano-titanium dioxide on the microstructure, mechanical properties and in vitro bioactivity of a freeze cast merwinite scaffold. Biotechnol. Prog. 2015, 31, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, Y.; Emadi, R.; Kharaziha, M.; Doostmohammadi, A. Reinforcement of electrospun poly(e-caprolactone) scaffold using diopside nanopowder to promote biological and physical properties. J. Appl. Polym. Sci. 2017, 134, 44433. [Google Scholar] [CrossRef]
- Shuai, C.; Liu, T.; Gao, C.; Feng, P.; Xiao, T.; Yu, K.; Peng, S. Mechanical and structural characterization of diopside scaffolds reinforced with graphene. J. Alloys Compd. 2016, 655, 86–92. [Google Scholar] [CrossRef]
- Teimouri, A.; Ghorbanian, L.; Dabirian, I. Preparation and characterization of silk/diopside composite nanofibers via electrospinning for tissue engineering application. Int. J. Chem. Mol. Eng. 2016, 10, 791–794. [Google Scholar]
- Arastouei, M.; Khodaei, M.; Atyabi, S.M.; Nodoushan, M.J. Improving the properties of the porous polylactic acid scaffold by akermanite nanoparticles for bone tissue engineering. J. Adv. Mater. Proc. 2020, 8, 11–19. [Google Scholar]
- Chen, C.; Watkins-Curry, P.; Smoak, M.; Hogan, K.; Deese, S.; McCandless, G.T.; Chan, J.Y.; Hayes, D.J. Targeting calcium magnesium silicates for polycaprolactone/ceramic composite scaffolds. ACS Biomater. Sci. Eng. 2015, 1, 94–102. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J.; Zhai, W.; Ni, S.; Wang, J. Porous akermanite scaffolds for bone tissue engineering: Preparation, characterization, and in vitro studies. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 78, 47–55. [Google Scholar] [CrossRef]
- Abdollahi, M.; Ghomi, H. Fabrication of highly porous merwinite scaffold using the space holder method. Int. J. Mater. Res. 2020, 111, 711–718. [Google Scholar] [CrossRef]
- Koppala, S.; John, S.P.; Balan, R.; Lokesh, B.; Munusamy, S.; Karthikeyan, P.; Godiya, C.B.; Chandragiri, S.Y.; Aminabhavi, T.M.; Duan, K.; et al. Glowing combustion synthesis, characterization and biomedical properties of Sr-hardystonite (Sr2ZnSi2O7) powders. Ceram. Int. 2022, 48, 23649–23656. [Google Scholar] [CrossRef]
- Jinga, S.I.; Constantinoiu, I.; Surdu, V.A.; Iordache, F.; Busuioc, C. Sol-gel-derived mineral scaffolds within SiO2–P2O5–CaO–MgO–ZnO–CaF2 system. J. Sol.-Gel. Sci. Technol. 2019, 90, 411–421. [Google Scholar] [CrossRef]
- Jinga, S.I.; Anghel, A.M.; Brincoveanu, S.F.; Bucur, R.M.; Florea, A.D.; Saftau, B.I.; Stroe, S.C.; Zamfirescu, A.I.; Busuioc, C. Ce/Sm/Sr-incorporating ceramic scaffolds obtained via sol-gel route. Materials 2021, 14, 1532. [Google Scholar] [CrossRef] [PubMed]
- Prefac, G.A.; Milea, M.L.; Vadureanu, A.M.; Muraru, S.; Dobrin, D.I.; Isopencu, G.O.; Jinga, S.I.; Raileanu, M.; Bacalum, M.; Busuioc, C. CeO2 containing thin films as bioactive coatings for orthopaedic implants. Coatings 2020, 10, 642. [Google Scholar] [CrossRef]
- Draghici, D.A.; Mihai, A.A.; Aioanei, M.O.; Negru, N.E.; Nicoara, A.I.; Jinga, S.I.; Miu, D.; Bacalum, M.; Busuioc, C. Strontium-substituted bioactive glass-ceramic films for tissue engineering. Bol. Soc. Esp. Ceram. Vidr. 2022, 61, 184–190. [Google Scholar] [CrossRef]
- Schitea, R.I.; Nitu, A.; Ciobota, A.A.; Munteanu, A.L.; David, I.M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed laser deposition derived bioactive glass-ceramic coatings for enhancing the biocompatibility of scaffolding materials. Materials 2020, 13, 2615. [Google Scholar] [CrossRef] [PubMed]









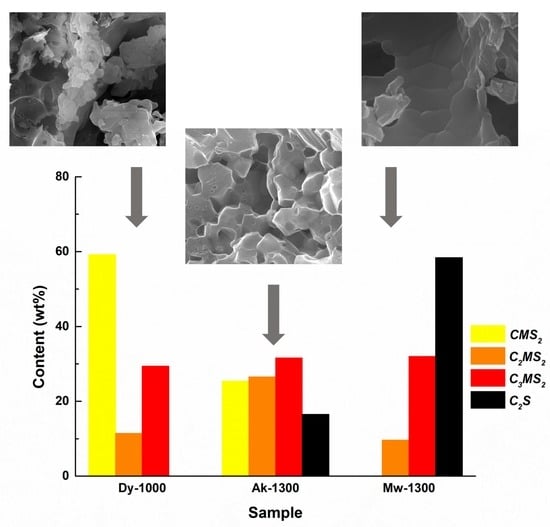
| Phases/Sample | CMS2 | C2MS2 | C3MS2 | C2S |
|---|---|---|---|---|
| (wt%) | ||||
| Dy-1000 | 59.2 | 11.4 | 29.4 | - |
| Ak-1300 | 25.4 | 26.5 | 31.6 | 16.5 |
| Mw-1300 | - | 9.6 | 32.0 | 58.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alecu, A.-E.; Costea, C.-C.; Surdu, V.-A.; Voicu, G.; Jinga, S.-I.; Busuioc, C. Processing of Calcium Magnesium Silicates by the Sol–Gel Route. Gels 2022, 8, 574. https://doi.org/10.3390/gels8090574
Alecu A-E, Costea C-C, Surdu V-A, Voicu G, Jinga S-I, Busuioc C. Processing of Calcium Magnesium Silicates by the Sol–Gel Route. Gels. 2022; 8(9):574. https://doi.org/10.3390/gels8090574
Chicago/Turabian StyleAlecu, Andrada-Elena, Claudiu-Constantin Costea, Vasile-Adrian Surdu, Georgeta Voicu, Sorin-Ion Jinga, and Cristina Busuioc. 2022. "Processing of Calcium Magnesium Silicates by the Sol–Gel Route" Gels 8, no. 9: 574. https://doi.org/10.3390/gels8090574
APA StyleAlecu, A. -E., Costea, C. -C., Surdu, V. -A., Voicu, G., Jinga, S. -I., & Busuioc, C. (2022). Processing of Calcium Magnesium Silicates by the Sol–Gel Route. Gels, 8(9), 574. https://doi.org/10.3390/gels8090574