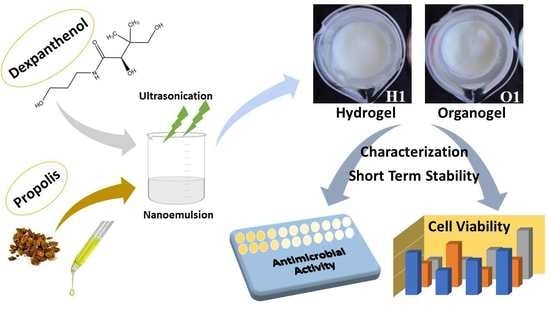
Nanoemulsion-Based Hydrogels and Organogels Containing Propolis and Dexpanthenol: Preparation, Characterization, and Comparative Evaluation of Stability, Antimicrobial, and Cytotoxic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of Quantification Method for Propolis and Dexpanthenol
2.2. Preparation of Nanoemulsions
2.3. Characterization of Nanoemulsions
2.4. Preparation of Hydrogels and Organogels
2.5. Characterization Studies of Hydrogels and Organogels
2.5.1. Organoleptic Characteristics
2.5.2. TEM Images
2.5.3. Drug Contents
2.5.4. pH and Gel-Sol Transition Temperature
2.5.5. Spreadability and Viscosity Analysis
2.5.6. FT-IR Analysis
2.5.7. In Vitro Release and Release Kinetics
2.6. Antimicrobial Properties of Hydrogels and Organogels
2.7. Cytotoxic Properties of Hydrogels and Organogels
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Development of Quantification Method for Propolis and Dexpanthenol
4.3. Preparation of Nanoemulsions
4.4. Characterization of Nanoemulsions
4.5. Preparation of Hydrogels and Organogels
4.5.1. Preparation of Nanoemulsion Based Hydrogels
4.5.2. Preparation of Nanoemulsion Based Organogels
4.6. Characterization Studies of Hydrogels and Organogels
4.6.1. Organoleptic Characteristics
4.6.2. TEM Images
4.6.3. Drug Contents
4.6.4. pH and Gel-Sol Transition Temperature
4.6.5. Spreadability and Viscosity Analysis
4.6.6. FT-IR Analysis
4.6.7. In Vitro Release and Release Kinetics
4.7. Antimicrobial Properties of Hydrogels and Organogels
4.8. Cytotoxic Properties of Hydrogels and Organogels
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Xu, X.; Fu, J.; Yu, D.G.; Liu, Y. Recent progress in electrospun polyacrylonitrile nanofiber-based wound dressing. Polymers 2022, 14, 3266. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Lai, F.; Offerta, A.; Sarpietro, M.G.; Micicchè, L.; Maccioni, A.; Valenti, D.; Fadda, A. From nanoemulsions to nanostructured lipid carriers: A relevant development in dermal delivery of drugs and cosmetics. J. Drug Deliv. Sci. Technol. 2015, 32, 100–112. [Google Scholar] [CrossRef]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Delivery 2010, 1, 109–131. [Google Scholar] [CrossRef]
- Neubert, R.H. Potentials of new nanocarriers for dermal and transdermal drug delivery. Eur. J. Pharm. Biopharm. 2011, 77, 1–2. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in nanotechnology-based delivery systems for dermal targeting of drugs: An enticing approach to offset psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef]
- Tayeb, H.H.; Sainsbury, F. Nanoemulsions in drug delivery: Formulation to medical application. Nanomedicine 2018, 13, 2507–2525. [Google Scholar] [CrossRef]
- Shehata, T.M.; Elnahas, H.M.; Elsewedy, H.S. Development, characterization and optimization of the anti-inflammatory influence of meloxicam loaded into a eucalyptus oil-based nanoemulgel. Gels 2022, 8, 262. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Kato, E.T.; Lobenberg, R.; Bou-Chacra, N.A. Challenges and future prospects of nanoemulsion as a drug delivery system. Curr. Pharm. Des. 2017, 23, 495–508. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef]
- Anand, K.; Ray, S.; Rahman, M.; Shaharyar, A.; Bhowmik, R.; Bera, R.; Karmakar, S. Nano-emulgel: Emerging as a smarter topical lipidic emulsion-based nanocarrier for skin healthcare applications. Recent Pat. Anti-Infect. Drug Discov. 2019, 14, 16–35. [Google Scholar] [CrossRef]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A promising phase in drug delivery. Curr. Pharm. Des. 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Mokhtar, M.; Hafez, S.; Mahdy, M. Organogels, hydrogels and bigels as transdermal delivery systems for diltiazem HCL. Asian J. Pharm. Sci. 2013, 8, 48–57. [Google Scholar]
- Shapiro, Y. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- de Lima, G.G.; de Souza, R.O.; Bozzi, A.D.; Poplawska, M.A.; Devine, D.M.; Nugent, M.J. Extraction method plays critical role in antibacterial activity of propolis-loaded hydrogels. J. Pharm. Sci. 2016, 105, 1248–1257. [Google Scholar] [CrossRef]
- Xie, M.; Liu, X.; Wang, S. Degradation of methylene blue through Fenton-like reaction catalyzed by MoS2-doped sodium alginate/Fe hydrogel. Colloids Surf. B Biointerfaces 2022, 214, 112443. [Google Scholar] [CrossRef]
- Wagh, V.D. Propolis: A wonder bees product and its pharmacological potentials. Adv. Pharm. Sci. 2013, 2013, 308249. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Gupta, R.K.; Stângaciu, Ş. Apitherapy: Holistic healing through the honeybee and bee products in countries with poor healthcare system. In Beekeeping for Poverty Alleviation and Livelihood Security, 1st ed.; Gupta, R., Reybroeck, W., Van Veen, J., Gupta, A., Eds.; Springer: Dordrecht, Switzerland, 2014; Volume 1, pp. 413–446. [Google Scholar]
- Demirdelen, S.; İmamoğlu, M.; Arslan, S.; Saygın, I. Topical dexpanthenol application improves healing of acute tympanic membrane perforations: An experimental study. ENT Updates 2016, 6, 116–120. [Google Scholar] [CrossRef]
- Ebner, F.; Heller, A.; Rippke, F.; Tausch, I. Topical use of dexpanthenol in skin disorders. Am. J. Clin. Derm. 2002, 6, 427–433. [Google Scholar] [CrossRef]
- Helaly, G.F.; El-Aziz, A.A.A.; Sonbol, F.; El-Banna, T.; Louise, N.L. Dexpanthenol and propolis extract in combination with local antibiotics for treatment of Staphylococcal and Pseudomonal wound infections. Arch. Clin. Microbiol. 2011, 2, 1–15. [Google Scholar]
- Maldonado, L.; Marcinkevicius, K.; Borelli, R.; Gennari, G.; Salomón, V.; Isla, M.; Vera, N.; Borelli, V. Differentiation of argentine propolis from different species of bees and geographical origins by UV spectroscopy and chemometric analysis. J. Saudi. Soc. Agric. Sci. 2020, 19, 185–191. [Google Scholar] [CrossRef]
- Fabris, S.; Bertelle, M.; Astafyeva, O.; Gregoris, E.; Zangrando, R.; Gambaro, A.; Lima, G.; Stevanato, R. Antioxidant properties and chemical composition relationship of europeans and brazilians propolis. Pharm. Pharm. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Poláček, R.; Kleinová, A.; Májek, P. Spectro-chemometric determination of panthenol enantiomeric excess in pharmaceutical products. Acta Chim. Slov. 2020, 13, 23–29. [Google Scholar] [CrossRef]
- Smail, S.S.; Ghareeb, M.M.; Omer, H.K.; Al-Kinani, A.A.; Alany, R.G. Studies on surfactants, cosurfactants, and oils for prospective use in formulation of ketorolac tromethamine ophthalmic nanoemulsions. Pharmaceutics 2021, 13, 467. [Google Scholar] [CrossRef]
- Malik, M.R.; Al-Harbi, F.F.; Nawaz, A.; Amin, A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N. Formulation and characterization of chitosan-decorated multiple nanoemulsion for topical delivery in vitro and ex vivo. Molecules 2022, 27, 3183. [Google Scholar] [CrossRef]
- Khani, S.; Keyhanfar, F.; Amani, A. Design and evaluation of oral nanoemulsion drug delivery system of mebudipine. Drug Deliv. 2016, 6, 2035–2043. [Google Scholar] [CrossRef]
- Akira, T.; Tomoko, M.; Shunichi, N.; Tadamichi, K. Effects of tween and span group emulsifiers on the stability of o/w emulsions. Chem. Pharm. Bull 1979, 27, 2921–2926. [Google Scholar]
- Syed Azhar, S.N.A.; Ashari, S.E.; Salim, N. Development of a kojic monooleate-enriched oil-in-water nanoemulsion as a potential carrier for hyperpigmentation treatment. Int. J. Nanomed. 2018, 13, 6465–6479. [Google Scholar] [CrossRef]
- Séguy, L.; Groo, A.C.; Goux, D.; Hennequin, D.; Malzert-Fréon, A. Design of non-haemolytic nanoemulsions for intravenous administration of hydrophobic APIs. Pharmaceutics 2020, 12, 1141. [Google Scholar] [CrossRef]
- Algahtani, M.; Ahmad, M.Z.; Nourein Hamed, I.; Albarqi, H.; Alyami, H.; Alyami, M.; Alqahtani, A.; Alasiri, A.; Algahtani, T.; Mohammed, A.; et al. Preparation and characterization of curcumin nanoemulgel utilizing ultrasonication technique for wound healing: In vitro, ex vivo, and in vivo evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Špaglová, M.; Čuchorová, M.; Čierna, M.; Poništ, S.; Bauerová, K. Microemulsions as solubilizers and penetration enhancers for minoxidil release from gels. Gels 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Hasssanzadeh, H.; Alizadeh, M.; Rezazad, B.M. Formulation of garlic oil-in-water nanoemulsion: Antimicrobial and physicochemical aspects. IET Nanobiotechnol. 2018, 12, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Almostafa, M.M.; Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Novel formulation of fusidic acid incorporated into a myrrh-oil-based nanoemulgel for the enhancement of skin bacterial infection treatment. Gels 2022, 8, 245. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Anwer, M.K.; Fatima, F.; Alali, A.S.; Kalam, M.A.; Zafar, A.; Alshehri, S.; Ghoneim, M.M. Development of apremilast nanoemulsion-loaded chitosan gels: In vitro evaluations and anti-inflammatory and wound healing studies on a rat model. Gels 2022, 8, 253. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Rahman, M.A.; Warsi, M.H.; Yusuf, M.; Alam, P. Development of nanogel loaded with lidocaine for wound-healing: Illustration of improved drug deposition and skin safety analysis. Gels 2022, 8, 466. [Google Scholar] [CrossRef]
- Tatsuko, H.; Junko, U.; Chika, Y.; Akira, K.; Hyoe, H. Gel–sol transition of poly(vinyl alcohol) hydrogels formed by freezing and thawing. Thermochim. Acta 2005, 431, 144–148. [Google Scholar]
- Kavya, G.; Jose, J. Development of amphotericin B Based organogels against mucocutaneous fungal infections. Braz J. Pharm. Sci. 2020, 56, e17509. [Google Scholar]
- Arora, R.; Aggarwal, G.; Harikumar, S.L.; Kaur, K. Nanoemulsion based hydrogel for enhanced transdermal delivery of ketoprofen. Adv. Pharm. J. 2014, 2014, 468456. [Google Scholar] [CrossRef]
- Sigmaaldrich. Available online: https://www.sigmaaldrich.com/TR/en/product/sigma/21904 (accessed on 8 November 2021).
- Lubrizol. Available online: https://www.lubrizol.com/-/media/Lubrizol/Health/TDS/TDS-730_Viscosity_Carbopol_in_Aqueous-Systems.pdf (accessed on 8 November 2021).
- Wu, Y.-W.; Sun, S.-Q.; Zhao, J.; Li, Y.; Zhou, Q. Rapid discrimination of extracts of Chinese propolis and poplar buds by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2008, 883, 48–54. [Google Scholar] [CrossRef]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Marek Kuś, P.; Jerković, I. Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, FTIR-ATR, UHPLC-DAD-QqTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef]
- Tuncay-Tanrıverdi, S.; Suat, B.; Azizoğlu, E.; Köse, F.A. In-vitro evaluation of dexpanthenol-loaded nanofiber mats for wound healing. Trop. J. Pharm. Res. 2018, 17, 387–394. [Google Scholar] [CrossRef]
- Tamizi, E.; Azizi, M.; Seyed Dorraji, M.S.; Dusti, Z.; Panahi-Azar, V. Stabilized core/shell PVA/SA nanofibers as an efficient drug delivery system for dexpanthenol. Polym. Bull. 2018, 75, 547–560. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Santos, J.D.; Monteiro, F.J. Development of a system to adsorb drugs onto calcium phosphate materials. J. Mater. Sci. Mater. Med. 2005, 16, 641–646. [Google Scholar] [CrossRef]
- Murei, A.; Ayinde, W.B.; Gitari, M.W.; Samie, A. Functionalization and antimicrobial evaluation of ampicillin, penicillin and vancomycin with pyrenacantha grandiflora baill and silver nanoparticles. Sci. Rep. 2020, 10, 11596. [Google Scholar]
- Rogowska, A.; Rafinska, K.; Pomastowski, P.; Walczak, J.; Railean-Plugaru, V.; Buszewska-Forajta, M.; Buszewski, B. silver nanoparticles functionalized with ampicillin. Electrophoresis 2017, 38, 2757–2764. [Google Scholar] [CrossRef]
- Nair, A.B.; Chaudhary, S.; Shah, H.; Jacob, S.; Mewada, V.; Shinu, P.; Shah, J. Intranasal delivery of darunavir-loaded mucoadhesive in situ gel: Experimental design, in vitro evaluation, and pharmacokinetic studies. Gels 2022, 8, 342. [Google Scholar] [CrossRef]
- Ghorpade, V.; Mali, K.; Dias, R.; Karande, P. Carbopol and Sodium Carboxymethylcellulose Based Methylsulfonylmethane Gels for Treatment of Osteoarthritis: In-vitro and In-vivo Evaluation. Ind. J. Pharm. Edu. Res. 2012, 46, 235–242. [Google Scholar]
- Camelo Pereira, S.R.; Franceschi, S.; Perez, E.; Girod Fullana, S.; Ré, M.I. Factors influencing the erosion rate and the drug release kinetics from organogels designed as matrices for oral controlled release of a hydrophobic drug. Drug Dev. Ind. Pharm. 2016, 42, 985–997. [Google Scholar] [CrossRef]
- Sevinç Özakar, R.; Özakar, E. Different biopolymers′ effects on the evaluation and characterization of floating tablets prepared by lyophilization technique to improve the quality control parameters. Polym. Korea 2022, 46, 145–158. [Google Scholar] [CrossRef]
- Lu, L.C.; Chen, Y.W.; Chou, C.C. Antibacterial activity of propolis against Staphylococcus aureus. Int. J. Food Microbiol. 2005, 102, 213–220. [Google Scholar] [CrossRef]
- Meto, A.; Colombari, B.; Meto, A.; Boaretto, G.; Pinetti, D.; Marchetti, L.; Benvenuti, S.; Pellati, F.; Blasi, E. Propolis Affects Pseudomonas aeruginosa Growth, Biofilm Formation, eDNA Release and Phenazine Production: Potential Involvement of Polyphenols. Microorganisms 2020, 8, 243. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kępa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A. In Vitro Antimicrobial Activity of Ethanolic Extract of Polish Propolis against Biofilm Forming Staphylococcus epidermidis Strains. Evid. Based Complement. Alternat. Med. 2013, 2013, 590703. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Fernandes Jr, A.; Lopes, C.A.; Bankova, V.; Funari, S.R. Seasonal effect on Brazilian propolis antibacterial activity. J. Ethnopharmacol. 2000, 73, 243–249. [Google Scholar] [CrossRef]
- Mencuci, R.; Favuzza, E.; Bottino, P.; Mazzantini, C.; Zanotton, E.; Pellegrini-Giampietro, D.E.; Landucci, E. A new ophthalmic formulation containing antiseptics and dexpanthenol: In vitro antimicrobial activity and effects on corneal and conjunctival epithelial cells. Exp. Eye Res. 2020, 201, 108269. [Google Scholar]
- Das, S.S.; Verma, P.R.P.; Singh, S.K. Screening and preparation of quercetin doped nanoemulsion: Characterizations, antioxidant and anti-bacterial activities. LWT—Food Sci. Technol. 2020, 124, 109141. [Google Scholar] [CrossRef]
- Gurpreet, K.; Singh, S.K. Review of Nanoemulsion Formulation and Characterization Techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Radice, S.; Kern, P.; Dietsch, H.; Mischler, S.; Michler, J. Methods for functionalization of microsized polystyrene beads with titania nanoparticles for cathodic electrophoretic deposition. J. Colloid Interface Sci. 2008, 318, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Sultan, M.H.; Javed, S.; Madkhali, O.A.; Alam, M.I.; Almoshari, Y.; Bakkari, M.A.; Sivadasan, D.; Salawi, A.; Jabeen, A.; Ahsan, W. Development and Optimization of Methylcellulose-Based Nanoemulgel Loaded with Nigella sativa Oil for Oral Health Management: Quadratic Model Approach. Molecules 2022, 27, 1796. [Google Scholar] [CrossRef]
- Andonova, V.Y.; Peneva, P.T.; Apostolova, E.G.; Dimcheva, T.D.; Peychev, Z.L.; Kassarova, M.I. Carbopol hydrogel/sorbitan monostearate-almond oil based organogel biphasic formulations: Preparation and characterization of the bigels. Trop. J. Pharm. Res. 2017, 16, 1455–1463. [Google Scholar] [CrossRef]
- Zidan, A.S.; Kamal, N.; Alayoubi, A.; Seggel, M.; İbrahim, S.; Rahman, Z.; Cruz, C.N.; Ashraf, M. Effect of Isopropyl Myristate on Transdermal Permeation of Testosterone From Ca.arbopol Gel. J. Pharm. Sci. 2017, 106, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.D.; Tamane, P.K.; Khante, N.; Pokharkar, V.B. QbD based optimization of curcumin nanoemulsion: DoE and cytotoxicity studies. Indian J. Pharm. Educ. Res. 2020, 54, 329–336. [Google Scholar] [CrossRef]
- Patel, D.; Patel, V. Development and characterization of pluronic lecithin organogel containing fluocinolone acetonide. Drug Dev. Ind. Pharm. 2021, 47, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ito, E.; Kida, R.; Hirose, K.; Noda, T.; Ozeki, T. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int. J. Pharm. 2021, 594, 120118. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Topal, G.R.; Kıymacı, M.E.; Özkan, Y. Preparation and in vitro characterization of vancomycin loaded PLGA nanoparticles for the treatment of Enterococcus faecalis infections. J. Fac. Pharm. Ank. 2022, 46, 350–363. [Google Scholar] [CrossRef]
- Pawar, H.A.; Dhavale, R. Development and Evaluation of Gastroretentive Floating Tablets of an Antidepressant Drug by Thermoplastic Granulation Technique. Appl. Spectrosc. Rev. 2014, 3, 122–132. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Sreedharan, S.M.; Singh, R. Ciprofloxacin Functionalized Biogenic Gold Nanoflowers as Nanoantibiotics Against Pathogenic Bacterial Strains. Int. J. Nanomed. 2019, 14, 9905–9916. [Google Scholar] [CrossRef] [Green Version]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of Mics and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 15 August 2022).










| Formulation Code | Propolis | Dexpanthenol | Tween 60 | Span 20 | Tween 20 | Span 80 |
|---|---|---|---|---|---|---|
| Nanoemulsion 1 | 25 | 250 | 100 | 100 | - | - |
| Nanoemulsion 2 | 25 | 250 | 200 | 100 | - | - |
| Nanoemulsion 3 | 25 | 250 | 100 | 200 | - | - |
| Nanoemulsion 4 | 25 | 250 | - | - | 100 | 100 |
| Nanoemulsion 5 | 25 | 250 | - | - | 200 | 100 |
| Nanoemulsion 6 | 25 | 250 | - | - | 100 | 200 |
| Formulation Code | Droplet Size (nm) * | Polydispersity Index * | Zeta Potential (mV) * | Conductivity (µS/cm) * | pH |
|---|---|---|---|---|---|
| Nanoemulsion 1 | 211.2 ± 6.45 | 0.173 ± 0.015 | −31.9 ± 0.52 | 17.00 ± 4.50 | 6.43 |
| Nanoemulsion 2 | 185.5 ± 3.30 | 0.205 ± 0.006 | −34.3 ± 0.31 | 15.40 ± 8.49 | 6.20 |
| Nanoemulsion 3 | 166.0 ± 0.97 | 0.149 ± 0.002 | −33.6 ± 1.55 | 12.10 ± 3.41 | 6.81 |
| Nanoemulsion 4 | 221.6 ± 4.71 | 0.189 ± 0.014 | −29.8 ± 0.45 | 16.90 ± 0.06 | 6.51 |
| Nanoemulsion 5 | 203.3 ± 0.84 | 0.216 ± 0.005 | −30.8 ± 0.83 | 12.00 ± 4.89 | 5.91 |
| Nanoemulsion 6 | 219.8 ± 4.05 | 0.207 ± 0.009 | −40.0 ± 1.42 | 7.20 ± 0.50 | 6.18 |
| Formulation Code | Phase Separation | Color | ||
|---|---|---|---|---|
| Room Temperature | Refrigerator Temperature | Room Temperature | Refrigerator Temperature | |
| Hydrogel 1 | - | - | Pale yellow to white | Pale yellow to white |
| Hydrogel 2 | - | - | Pale yellow to white | Pale yellow to white |
| Hydrogel 3 | - | - | Pale yellow to white | Pale yellow to white |
| Hydrogel 4 | + | - | Pale yellow | Pale yellow to white |
| Hydrogel 5 | + | - | Pale yellow | Pale yellow to white |
| Hydrogel 6 | + | - | Pale yellow | Pale yellow to white |
| Organogel 1 | - | - | Pale yellow to white | Pale yellow to white |
| Organogel 2 | - | - | Pale yellow to white | Pale yellow to white |
| Organogel 3 | - | - | Pale yellow to white | Pale yellow to white |
| Organogel 4 | - | - | Pale yellow to white | Pale yellow to white |
| Organogel 5 | - | - | Pale yellow to white | Pale yellow to white |
| Organogel 6 | + | - | Pale yellow | Pale yellow to white |
| Formulation Code | Freshly Prepared | After 3 Months Room Temperature | After 3 Months Refrigerator Temperature | |||
|---|---|---|---|---|---|---|
| Propolis | Dexpanthenol | Propolis | Dexpanthenol | Propolis | Dexpanthenol | |
| Hydrogel 1 | 102.06 ± 2.67 | 101.68 ± 0.68 | 102.00 ± 0.47 | 100.01 ± 0.63 | 102.23 ± 1.20 | 99.91 ± 0.85 |
| Hydrogel 2 | 101.35 ± 3.13 | 100.18 ± 1.10 | 102.70 ± 0.47 | 99.04 ± 0.57 | 99.48 ± 1.30 | 99.69 ± 0.83 |
| Hydrogel 3 | 100.24 ± 0.63 | 102.76 ± 1.87 | 99.54 ± 1.73 | 99.91 ± 0.98 | 102.06 ± 0.54 | 101.46 ± 1.31 |
| Hydrogel 4 | 99.60 ± 1.17 | 101.26 ± 2.80 | - | - | 99.89 ± 0.54 | 99.99 ± 1.02 |
| Hydrogel 5 | 100.05 ± 1.90 | 100.48 ± 4.42 | - | - | 102.29 ± 0.79 | 98.98 ± 1.49 |
| Hydrogel 6 | 101.82 ± 0.98 | 99.76 ± 1.19 | - | - | 100.89 ± 1.54 | 101.36 ± 1.57 |
| Organogel 1 | 99.89 ± 0.53 | 99.62 ± 1.46 | 102.82 ± 1.24 | 101.48 ± 0.95 | 101.12 ± 1.15 | 99.23 ± 0.82 |
| Organogel 2 | 99.54 ± 2.10 | 98.15 ± 0.85 | 100.94 ± 1.10 | 99.78 ± 1.82 | 102.88 ± 2.03 | 99.55 ± 1.23 |
| Organogel 3 | 100.59 ± 1.43 | 101.08 ± 1.36 | 103.47 ± 1.74 | 99.76 ± 1.74 | 100.71 ± 2.82 | 101.49 ± 1.51 |
| Organogel 4 | 100.06 ± 1.69 | 99.98 ± 1.36 | 102.88 ± 0.30 | 101.12 ± 1.29 | 101.06 ± 1.86 | 98.92 ± 1.72 |
| Organogel 5 | 99.89 ± 1.03 | 98.98 ± 1.02 | 102.70 ± 1.96 | 100.08 ± 1.67 | 102.18 ± 1.15 | 100.49 ± 1.26 |
| Organogel 6 | 99.01 ± 1.84 | 100.05 ± 1.79 | - | - | 99.83 ± 1.00 | 100.42 ± 0.74 |
| Formulation Code | pH | Gel-Sol Transition Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| Freshly Prepared | After 3 Months Room Temperature | After 3 Months Refrigerator Temperature | Freshly Prepared | After 3 Months Room Temperature | After 3 Months Refrigerator Temperature | |
| Hydrogel 1 | 6.27 | 6.15 | 6.45 | 85 | 90 | 90 |
| Hydrogel 2 | 6.25 | 6.17 | 6.05 | 90 | 90 | 90 |
| Hydrogel 3 | 5.80 | 6.10 | 6.54 | 90 | 80 | 90 |
| Hydrogel 4 | 5.99 | 6.13 | 6.16 | 85 | - | 90 |
| Hydrogel 5 | 5.85 | 5.86 | 6.01 | 90 | - | 90 |
| Hydrogel 6 | 6.20 | 5.95 | 5.76 | 90 | - | 90 |
| Organogel 1 | 4.56 | 4.72 | 5.06 | 85 | 90 | 90 |
| Organogel 2 | 4.51 | 4.52 | 4.74 | 85 | 95 | 85 |
| Organogel 3 | 4.99 | 4.89 | 4.72 | 90 | 95 | 90 |
| Organogel 4 | 4.62 | 4.78 | 4.63 | 85 | 95 | 95 |
| Organogel 5 | 4.65 | 4.71 | 4.43 | 85 | 90 | 80 |
| Organogel 6 | 4.52 | 4.54 | 4.35 | 90 | - | 85 |
| Spreadability | g·cm/s Diameter ± SD | Viscosity (cP) | |||||
|---|---|---|---|---|---|---|---|
| Formulation Code | Freshly Prepared | After 3 Months Room Temperature | After 3 Months Refrigerator Temperature | Freshly Prepared | After 3 Months Room Temperature | After 3 Months Refrigerator Temperature | |
| Hydrogel 1 | 15.66 ± 0.59 26.1 ± 1.0 mm | 15.24 ± 0.08 25.4 ± 0.1 mm | 19.23 ± 0.30 32.1 ± 0.5 mm | 2528.7 ± 168.5 | 1706.7 ± 48.6 | 2585.7 ± 156.3 | |
| Hydrogel 2 | 24.42 ± 0.51 40.7 ± 0.8 mm | 23.13 ± 0.55 38.6 ± 0.9 mm | 17.31 ± 0.30 28.9 ± 0.5 mm | 3479.0 ± 123.0 | 2533.3 ± 144.6 | 3425.7 ± 124.5 | |
| Hydrogel 3 | 15.00 ± 1.02 25.0 ± 1.7 mm | 14.88 ± 0.25 24.8 ± 0.4 mm | 21.63 ± 0.30 36.1 ± 0.5 mm | 3690.7 ± 197.8 | 2792.7 ± 119.1 | 3816.7 ± 106.5 | |
| Hydrogel 4 | 24.99 ± 0.89 41.7 ± 1.5 mm | - | 24.27 ± 0.38 40.5 ± 0.6 mm | 3442.0 ± 163.7 | - | 3431.3 ± 104.6 | |
| Hydrogel 5 | 15.63 ± 1.32 26.1 ± 2.2 mm | - | 15.12 ± 0.17 25.2 ± 0.3 mm | 3350.7 ± 142.6 | - | 3291.7 ± 202.0 | |
| Hydrogel 6 | 23.88 ± 0.17 39.8 ± 0.3 mm | - | 21.15 ± 0.13 35.3 ± 0.2 mm | 3122.0 ± 250.6 | - | 3174.3 ± 114.7 | |
| Organogel 1 | 17.67 ± 0.13 29.5 ± 0.2 mm | 14.64 ± 0.08 24.4 ± 0.1 mm | 15.09 ± 0.13 25.2 ± 0.2 mm | 1364.7 ± 100.2 | 1859.3 ± 92.8 | 1324.7 ± 56.8 | |
| Organogel 2 | 23.97 ± 1.15 40.0 ± 1.9 mm | 30.93 ± 1.32 51.6 ± 2.2 mm | 29.58 ± 0.51 49.3 ± 0.8 mm | 1891.3 ± 71.9 | 1312.7 ± 127.5 | 1655.7 ± 129.0 | |
| Organogel 3 | 17.73 ± 0.21 29.6 ± 0.4 mm | 14.70 ± 0.25 24.5 ± 0.4 mm | 14.73 ± 0.98 24.6 ± 1.6 mm | 1977.3 ± 172.7 | 996.0 ± 153.2 | 1827.3 ± 110.4 | |
| Organogel 4 | 23.43 ± 0.72 39.1 ± 1.2 mm | 28.08 ± 2.21 46.8 ± 3.7 mm | 28.08 ± 0.42 46.8 ± 0.7 mm | 952.6 ± 135.1 | 143.9 ± 6.6 | 814.2 ± 125.1 | |
| Organogel 5 | 18.12 ± 0.08 30.2 ± 0.1 mm | 14.97 ± 0.72 25.0 ± 1.2 mm | 14.97 ± 0.04 25.0 ± 0.1 mm | 1026.7 ± 121.1 | 344.5 ± 36.0 | 1085.0 ± 142.1 | |
| Organogel 6 | 23.76 ± 0.08 39.6 ± 0.1 mm | - | 28.50 ± 0.59 47.5 ± 1.0 mm | 1342.0 ± 115.4 | - | 1194.3 ± 92.9 | |
| Formulation Code | Zero Order | First Order | Higuchi | Korsmeyer–Peppas | Release Mechanism | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | ||
| Propolis Hydrogel 1 | 0.989 | 0.644 | 0.987 | 0.886 | 1.512 | Super Case-II Transport |
| Propolis Organogel 1 | 0.995 | 0.738 | 0.983 | 0.945 | 1.231 | Super Case-II Transport |
| Propolis Nanoemulsion 1 | 0.995 | 0.589 | 0.970 | 0.826 | 1.749 | Super Case-II Transport |
| Pure Propolis | 0.987 | 0.604 | 0.994 | 0.868 | 1.802 | Super Case-II Transport |
| Dexpanthenol Hydrogel 1 | 0.958 | 0.547 | 0.993 | 0.836 | 1.820 | Super Case-II Transport |
| Dexpanthenol Organogel 1 | 0.944 | 0.512 | 0.992 | 0.808 | 1.813 | Super Case-II Transport |
| Dexpanthenol Nanoemulsion 1 | 0.965 | 0.499 | 0.996 | 0.792 | 2.131 | Super Case-II Transport |
| Pure Dexpanthenol | 0.966 | 0.510 | 0.995 | 0.805 | 2.208 | Super Case-II Transport |
| Formulation Code | S. aureus | S. epidermidis | E. coli | P. aeruginosa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 µL | 50 µL | 100 µL | 25 µL | 50 µL | 100 µL | 25 µL | 50 µL | 100 µL | 25 µL | 50 µL | 100 µL | |
| Blank Hydrogel | - | - | - | - | - | - | - | - | - | - | - | - |
| Hydrogel 1 | - | 9 | 11 | - | - | - | - | - | - | - | - | - |
| Blank Organogel | - | - | - | - | - | - | - | - | - | - | - | - |
| Organogel 1 | - | - | 8 | 8 | 12 | 12 | - | - | - | - | - | - |
| Pure Propolis | - | - | - | - | - | 8 | - | - | - | - | - | - |
| Pure Dexpanthenol | 12 | 20 | 22 | - | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevinç-Özakar, R.; Seyret, E.; Özakar, E.; Adıgüzel, M.C. Nanoemulsion-Based Hydrogels and Organogels Containing Propolis and Dexpanthenol: Preparation, Characterization, and Comparative Evaluation of Stability, Antimicrobial, and Cytotoxic Properties. Gels 2022, 8, 578. https://doi.org/10.3390/gels8090578
Sevinç-Özakar R, Seyret E, Özakar E, Adıgüzel MC. Nanoemulsion-Based Hydrogels and Organogels Containing Propolis and Dexpanthenol: Preparation, Characterization, and Comparative Evaluation of Stability, Antimicrobial, and Cytotoxic Properties. Gels. 2022; 8(9):578. https://doi.org/10.3390/gels8090578
Chicago/Turabian StyleSevinç-Özakar, Rukiye, Emrah Seyret, Emrah Özakar, and Mehmet Cemal Adıgüzel. 2022. "Nanoemulsion-Based Hydrogels and Organogels Containing Propolis and Dexpanthenol: Preparation, Characterization, and Comparative Evaluation of Stability, Antimicrobial, and Cytotoxic Properties" Gels 8, no. 9: 578. https://doi.org/10.3390/gels8090578
APA StyleSevinç-Özakar, R., Seyret, E., Özakar, E., & Adıgüzel, M. C. (2022). Nanoemulsion-Based Hydrogels and Organogels Containing Propolis and Dexpanthenol: Preparation, Characterization, and Comparative Evaluation of Stability, Antimicrobial, and Cytotoxic Properties. Gels, 8(9), 578. https://doi.org/10.3390/gels8090578