Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery
Abstract
:1. Introduction
2. Results and Discussion
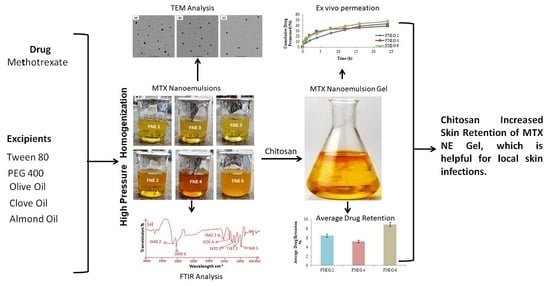
2.1. ATR FTIR Study
2.2. Thermodynamic Stability Analysis of Nanoemulsion Formulations
2.3. Organoleptic Analysis of Prepared Nanoemulsion
2.4. Particle Size, Polydispersity Index, and Zeta Potential
2.5. Entrapment Efficiency and Drug Content Evaluation
2.6. Transmission Electron Microscopy
2.7. Preparation of Methotrexate Nanoemulsion Gel
2.8. Characterization of MTX-Loaded Nanoemulsion Gel Formulations
2.8.1. pH, Rheological Behavior, and Assay of Gel Formulations
2.8.2. Spreadability
2.8.3. Extrudability and Drug Content
2.9. Skin Irritation Test
2.10. In Vitro Drug Release Study
2.11. Drug Release Kinetics
2.12. In Vitro Skin Permeation of Methotrexate-loaded Nanoemulsion Gel Formulations
2.13. Drug Retention Analysis
2.14. In Vivo Studies
2.15. Stability Determination
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Methotrexate O/W Nanoemulsion
4.3. ATR FTIR Study
4.4. Thermodynamic Stability Analysis of Optimized Nanoemulsion Formulations
4.5. Characterization of Prepared Methotrexate Nanoemulsion Formulations
4.5.1. Size, Polydispersity Index, and Zeta Potential
4.5.2. Physicochemical Assessment of Nanoemulsion Preparations
4.5.3. Entrapment Efficiency (EE)
4.5.4. Nanoemulsion Transmission Electron Microscopy
4.6. Preparation of Methotrexate Nanoemulsion Gel
4.7. Characterization of Nanoemulsion Gel Formulations
4.7.1. Organoleptic Appearance and Homogeneity
4.7.2. pH and Rheological Study
4.7.3. Spreadability
4.7.4. Extrudability
4.7.5. Drug Content
4.8. Skin Irritation Test
4.9. In Vitro Release and Kinteic Profiling
4.10. Ex Vivo Permeation
4.11. Skin Drug Retention Analysis
4.12. In Vivo Studies
4.13. Stability Studies
4.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latif, M.S.; Al-Harbi, F.F.; Nawaz, A.; Rashid, S.A.; Farid, A.; Al Mohaini, M.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N. Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and In Vivo Characterization. Polymers 2022, 14, 1310. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, F.; Kim, B.S. Recent advances in polymeric transdermal drug delivery systems. J. Control. Release 2021, 341, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef]
- Das Kurmi, B.; Tekchandani, P.; Paliwal, R.; Paliwal, S.R. Transdermal Drug Delivery: Opportunities and Challenges for Controlled Delivery of Therapeutic Agents Using Nanocarriers. Curr. Drug Metab. 2017, 18, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Ghosalkar, S.; Singh, P.; Ravikumar, P. Emerging topical drug delivery approaches for the treatment of Atopic dermatitis. J. Cosmet. Dermatol. 2021, 21, 536–549. [Google Scholar] [CrossRef]
- Chakole, C.M.; Chauhan, M.K. Research progress of nanostructured lipid carriers in ocular drug delivery. Drug Deliv. Lett. 2021, 11, 203–219. [Google Scholar] [CrossRef]
- Shang, H.; Younas, A.; Zhang, N. Recent advances on transdermal delivery systems for the treatment of arthritic injuries: From classical treatment to nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1778. [Google Scholar] [CrossRef]
- Srivastava, S.; Rasool, M. Underpinning IL-6 biology and emphasizing selective JAK blockade as the potential alternate therapeutic intervention for rheumatoid arthritis. Life Sci. 2022, 298, 120516. [Google Scholar] [CrossRef]
- Zeien, J.; Qiu, W.; Triay, M.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Clinical implications of chemotherapeutic agent organ toxicity on perioperative care. Biomed. Pharmacother. 2021, 146, 112503. [Google Scholar] [CrossRef]
- Damiani, G.; Pacifico, A.; Linder, D.M.; Pigatto, P.D.; Conic, R.; Grada, A.; Bragazzi, N.L. Nanodermatology-based solutions for psoriasis: State-of-the art and future prospects. Dermatol. Ther. 2019, 32, e13113. [Google Scholar] [CrossRef]
- Gushiken, L.; Beserra, F.; Bastos, J.; Jackson, C.; Pellizzon, C. Cutaneous Wound Healing: An Update from Physiopathology to Current Therapies. Life 2021, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Alhasso, B.; Ghori, M.U.; Conway, B.R. Systematic Review on the Effectiveness of Essential and Carrier Oils as Skin Penetration Enhancers in Pharmaceutical Formulations. Sci. Pharm. 2022, 90, 14. [Google Scholar] [CrossRef]
- Nunes, A.; Gonçalves, L.; Marto, J.; Martins, A.; Silva, A.; Pinto, P.; Martins, M.; Fraga, C.; Ribeiro, H. Investigations of Olive Oil Industry By-Products Extracts with Potential Skin Benefits in Topical Formulations. Pharmaceutics 2021, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, K. Effect of Olive Oil Acidity on Skin Delivery of Diclofenac: In vitro Evaluation and ex vivo Skin Permeability Studies. J. Biomed. Nanotechnol. 2022, 18, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 2020, 11, 785. [Google Scholar] [CrossRef]
- Teaima, M.H.; Alsofany, J.M.; El-Nabarawi, M.A. Clove Oil Endorsed Transdermal Flux of Dronedarone Hydrochloride Loaded Bilosomal Nanogel: Factorial Design, In vitro Evaluation and Ex vivo Permeation. AAPS PharmSciTech 2022, 23, 1–14. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Ojha, B.; Jain, V.K.; Gupta, S.; Talegaonkar, S.; Jain, K. Nanoemulgel: A promising novel formulation for treatment of skin ailments. Polym. Bull. 2021, 79, 4441–4465. [Google Scholar] [CrossRef]
- Parekh, K.; A Mehta, T.; Dhas, N.; Kumar, P.; Popat, A. Emerging Nanomedicines for the Treatment of Atopic Dermatitis. AAPS PharmSciTech 2021, 22, 55. [Google Scholar] [CrossRef]
- Harshitha, V.; Swamy, M.V.; Kumar, D.P.; Rani, K.S.; Trinath, A. Nanoemulgel: A process promising in drug delivery system. Res. J. Pharm. Dos. Technol. 2020, 12, 125–130. [Google Scholar] [CrossRef]
- Saidi, M.; Dabbaghi, A.; Rahmani, S. Swelling and drug delivery kinetics of click-synthesized hydrogels based on various combinations of PEG and star-shaped PCL: Influence of network parameters on swelling and release behavior. Polym. Bull. 2019, 77, 3989–4010. [Google Scholar] [CrossRef]
- Tungadi, R.; Susanty, W.; Wicita, P.; Pido, E. Transdermal Delivery of Snakehead Fish (Ophiocephalus striatus) Nanoemulgel Containing Hydrophobic Powder for Burn Wound. Pharm. Sci. 2018, 24, 313–323. [Google Scholar] [CrossRef]
- Khatoon, K.; Ali, A.; Ahmad, F.J.; Hafeez, Z.; Rizvi, M.; Akhter, S.; Beg, S. Novel nanoemulsion gel containing triple natural bio-actives combination of curcumin, thymoquinone, and resveratrol improves psoriasis therapy: In vitro and in vivo studies. Drug Deliv. Transl. Res. 2021, 11, 1245–1260. [Google Scholar] [CrossRef]
- Kassem, A.A.; Salama, A.; Mohsen, A.M. Formulation and optimization of cationic nanoemulsions for enhanced ocular delivery of dorzolamide hydrochloride using Box-Behnken design: In vitro and in vivo assessments. J. Drug Deliv. Sci. Technol. 2022, 68, 103047. [Google Scholar] [CrossRef]
- Patel, D.; Patel, B.; Thakkar, H. Lipid Based Nanocarriers: Promising Drug Delivery System for Topical Application. Eur. J. Lipid Sci. Technol. 2021, 123, 2000264. [Google Scholar] [CrossRef]
- Zeb, A.; Qureshi, O.S.; Yu, C.-H.; Akram, M.; Kim, H.-S.; Kim, M.-S.; Kang, J.-H.; Majid, A.; Chang, S.-Y.; Bae, O.-N.; et al. Enhanced anti-rheumatic activity of methotrexate-entrapped ultradeformable liposomal gel in adjuvant-induced arthritis rat model. Int. J. Pharm. 2017, 525, 92–100. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Zahid, F.; Batool, S.; Ud-Din, F.; Ali, Z.; Nabi, M.; Khan, S.; Salman, O.; Khan, G.M. Antileishmanial Agents Co-loaded in Transfersomes with Enhanced Macrophage Uptake and Reduced Toxicity. AAPS PharmSciTech 2022, 23, 226. [Google Scholar] [CrossRef]
- Jyothi, V.G.S.; Ghouse, S.M.; Khatri, D.K.; Nanduri, S.; Singh, S.B.; Madan, J. Lipid nanoparticles in topical dermal drug delivery: Does chemistry of lipid persuade skin penetration? J. Drug Deliv. Sci. Technol. 2022, 69, 103176. [Google Scholar] [CrossRef]
- Aleanizy, F.S.; Taha, E.I.; Salem-Bekhit, M.M.; Felimban, A.M.J.; Al-Suwayeh, S.A.; Al-Joufi, F.A.; Muharram, M.M.; Alqahtani, F.Y.; Shakeel, F.; Youssof, A.M.E.; et al. Formulation and in vitro and in vivo evaluation of surfactant-stabilized mucoadhesive nanogels for vaginal delivery of fluconazole. Drug Dev. Ind. Pharm. 2021, 47, 1935–1942. [Google Scholar] [CrossRef]
- Rajitha, P.; Shammika, P.; Aiswarya, S.; Gopikrishnan, A.; Jayakumar, R.; Sabitha, M. Chaulmoogra oil based methotrexate loaded topical nanoemulsion for the treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2019, 49, 463–476. [Google Scholar] [CrossRef]
- Karadurmus, L.; Corman, M.E.; Uzun, L.; Ozkan, S.A. Enantioselective recognition of esomeprazole with a molecularly imprinted sol–gel-based electrochemical sensor. Mikrochim. Acta 2022, 189, 225. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.A.; Bashir, S.; Naseem, F.; Farid, A.; Rather, I.A.; Hakeem, K.R. Olive Oil Based Methotrexate Loaded Topical Nanoemulsion Gel for the Treatment of Imiquimod Induced Psoriasis-like Skin Inflammation in an Animal Model. Biology 2021, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.; Tandon, R.; Kalia, A.; Mahajan, B.V.C. Nanoemulsion Formulation of Ocimum gratissimum Essential Oil and Its Antifungal Activity Against Penicillium digitatum. J. Nanosci. Nanotechnol. 2021, 21, 3556–3565. [Google Scholar] [CrossRef] [PubMed]
- Abedinpour, N.; Ghanbariasad, A.; Taghinezhad, A.; Osanloo, M. Preparation of Nanoemulsions of Mentha piperita Essential Oil and Investigation of Their Cytotoxic Effect on Human Breast Cancer Lines. Bionanoscience 2021, 11, 428–436. [Google Scholar] [CrossRef]
- Chrastina, A.; Welsh, J.; Borgström, P.; Baron, V.T. Propylene Glycol Caprylate-Based Nanoemulsion Formulation of Plumbagin: Development and Characterization of Anticancer Activity. BioMed Res. Int. 2022, 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.K.; Muhialdin, B.J.; Hussin, A.S.M. Characterization of nanoemulsion of Nigella sativa oil and its application in ice cream. Food Sci. Nutr. 2020, 8, 2608–2618. [Google Scholar] [CrossRef] [Green Version]
- Rashid, S.A.; Bashir, S.; Ullah, H.; ullah Shah, K.; Khan, D.H.; Shah, P.A.; Danish, M.Z.; Khan, M.H.; Mahmood, S.; Sohaib, M.; et al. Development, characterization and optimization of methotrexate-olive oil nano-emulsion for topical application. Pak. J. Pharm. Sci. 2021, 34, 205–215. [Google Scholar]
- Nawaz, A.; Latif, M.S.; Alnuwaiser, M.A.; Ullah, S.; Iqbal, M.; Alfatama, M.; Lim, V. Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery. Gels 2022, 8, 412. [Google Scholar] [CrossRef]
- Poonia, N.; Lather, V.; Kaur, B.; Kirthanashri, S.V.; Pandita, D. Optimization and Development of Methotrexate- and Resveratrol-Loaded Nanoemulsion Formulation Using Box–Behnken Design for Rheumatoid Arthritis. ASSAY Drug Dev. Technol. 2020, 18, 356–368. [Google Scholar] [CrossRef]
- Khan, M.K.; Khan, B.A.; Uzair, B.; Niaz, S.I.; Khan, H.; Hosny, K.M.; Menaa, F. Development of Chitosan-Based Nanoemulsion Gel Containing Microbial Secondary Metabolite with Effective Antifungal Activity: In vitro and in vivo Characterizations. Int. J. Nanomed. 2021, 16, 8203–8219. [Google Scholar] [CrossRef] [PubMed]
- Rani, K.R.V.; Rajan, S.; Bhupathyraaj, M.; Priya, R.K.; Halligudi, N.; Al-Ghazali, M.A.; Sridhar, S.B.; Shareef, J.; Thomas, S.; Desai, S.M.; et al. The Effect of Polymers on Drug Release Kinetics in Nanoemulsion In Situ Gel Formulation. Polymers 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Sevinç-Özakar, R.; Seyret, E.; Özakar, E.; Adıgüzel, M.C. Nanoemulsion-Based Hydrogels and Organogels Containing Propolis and Dexpanthenol: Preparation, Characterization, and Comparative Evaluation of Stability, Antimicrobial, and Cytotoxic Properties. Gels 2022, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Burki, I.K.; Khan, M.K.; Khan, B.A.; Uzair, B.; Braga, V.A.; Jamil, Q.A. Formulation Development, Characterization, and Evaluation of a Novel Dexibuprofen-Capsaicin Skin Emulgel with Improved In Vivo Anti-inflammatory and Analgesic Effects. AAPS PharmSciTech 2020, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Mulleria, S.S.; Marina, K.; Ghetia, S.M. Formulation, Optimization and in vitro Evaluation of Apremilast Nanoemulgel for Topical Delivery. Int. J. Pharm. Investig. 2021, 11, 230–237. [Google Scholar] [CrossRef]
- Nawaz, A.; Farid, A.; Safdar, M.; Latif, M.S.; Ghazanfar, S.; Akhtar, N.; Al Jaouni, S.K.; Selim, S.; Khan, M.W. Formulation Development and Ex-Vivo Permeability of Curcumin Hydrogels under the Influence of Natural Chemical Enhancers. Gels 2022, 8, 384. [Google Scholar] [CrossRef]
- Valizadeh, A.; Shirzad, M.; Pourmand, M.R.; Farahmandfar, M.; Sereshti, H.; Amani, A. Levofloxacin nanoemulsion gel has a powerful healing effect on infected wound in streptozotocin-induced diabetic rats. Drug Deliv. Transl. Res. 2020, 11, 292–304. [Google Scholar] [CrossRef]
- Eze, C.C.; Ekeke, N.; Alphonsus, C.; Lehman, L.; Chukwu, J.N.; Nwafor, C.C.; Stillwaggon, E.; Meka, A.O.; Sawers, L.; Ikebudu, J.; et al. Effectiveness of self-care interventions for integrated morbidity management of skin neglected tropical diseases in Anambra State, Nigeria. BMC Public Health 2021, 21, 1748. [Google Scholar] [CrossRef]
- Latif, M.S.; Nawaz, A.; Rashid, S.A.; Akhlaq, M.; Iqbal, A.; Khan, M.J.; Khan, M.S.; Lim, V.; Alfatama, M. Formulation of Polymers-Based Methotrexate Patches and Investigation of the Effect of Various Penetration Enhancers: In Vitro, Ex Vivo and In Vivo Characterization. Polymers 2022, 14, 2211. [Google Scholar] [CrossRef]
- Alam Shah, M.K.; Azad, A.K.; Nawaz, A.; Ullah, S.; Latif, M.S.; Rahman, H.; Alsharif, K.F.; Alzahrani, K.J.; El-Kott, A.F.; Albrakati, A.; et al. Formulation Development, Characterization and Antifungal Evaluation of Chitosan NPs for Topical Delivery of Voriconazole In Vitro and Ex Vivo. Polymers 2021, 14, 135. [Google Scholar] [CrossRef]









| F.Code | Characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Color | Odor | Phase Separation | Centrifugation | Thermodynamics | ||||||
| 4 ± 2 °C | 40 ± 2 °C | 4 ± 2 °C | 40 ± 2 °C | 4 ± 2 °C | 40 ± 2 °C | 4 ± 2 °C | 40 ± 2 °C | 4 ± 2 °C | 40 ± 2 °C | |
| FNE1 | Pale yellow | Pale yellow | No change | No change | No | No | Stable | Stable | Passed | Passed |
| FNE2 | Pale yellow | Pale yellow | No change | No change | No | No | Stable | Stable | Passed | Passed |
| FNE3 | Pale yellow | Pale yellow | No change | No change | No | No | Stable | Stable | Passed | Passed |
| FNE4 | Dark brown | Dark brown | No change | No change | No | No | Stable | Stable | Passed | Passed |
| FNE5 | Pale yellow | Pale yellow | No change | No change | No | No | Stable | Stable | Passed | Passed |
| FNE6 | Light brown | Light brown | No change | No change | No | No | Stable | Stable | Passed | Passed |
| Parameters | FNE1 | FNE2 | FNE3 | FNE4 | FNE5 | FNE6 |
|---|---|---|---|---|---|---|
| Physical Appearance | Transparent | Transparent | Transparent | Transparent | Transparent | Transparent |
| Clarity | Clear | Clear | Clear | Clear | Clear | Clear |
| pH | 5.48 ± 0.41 | 5.72 ± 0.21 | 5.51 ± 0.32 | 5.63 ± 0.25 | 5.59 ± 0.13 | 5.64 ± 0.44 |
| Homogeneity | Excellent | Excellent | Excellent | Excellent | Excellent | Excellent |
| F.Code | Particle Size | PDI | Zeta Potential (mV) | Entrapment Efficiency (%) | Drug Content (%) |
|---|---|---|---|---|---|
| FNE1 | 18.71 ± 3.45 | 0.386 ± 0.03 | −11.6 ± 0.15 | 0.0 | 0.0 |
| FNE2 | 20.81 ± 2.87 | 0.617 ± 0.01 | −9.33 ± 0.24 | 76.87 ± 1.9 | 87.2 ± 0.71 |
| FNE3 | 28.83 ± 4.23 | 0.732 ± 0.04 | −13.4 ± 0.22 | 0.0 | 0.0 |
| FNE4 | 17.52 ± 5.13 | 0.391 ± 0.02 | −8.59 ± 0.17 | 75.38 ± 2.3 | 86.1 ± 0.36 |
| FNE5 | 16.76 ± 2.48 | 0.251 ± 0.04 | −13.2 ± 0.43 | 0.0 | 0.0 |
| FNE6 | 28.58 ± 4.31 | 0.699 ± 0.03 | −9.44 ± 0.52 | 78.12 ± 1.6 | 91.5 ± 0.52 |
| F. Code | pH | Viscosity (Centipoise) | Spreadability (g cm/s) | Extrudability | Drug Content (%) | Skin Irritation |
|---|---|---|---|---|---|---|
| FNEG2 | 5.42 ± 0.25 | 9986 ± 13.5 | 20.13 ± 1.23 | 88.27 ± 0.54 | 93.12 ± 0.45 | Nil |
| FNEG4 | 5.61 ± 0.31 | 9843 ± 12.3 | 20.82 ± 1.45 | 85.43 ± 0.34 | 95.36 ± 0.52 | Nil |
| FNEG6 | 5.53 ± 0.42 | 9812 ± 13.1 | 21.46 ± 1.65 | 87.15 ± 0.27 | 94.61 ± 0.63 | Nil |
| F.Code | Korsmeyer Model | |||
|---|---|---|---|---|
| K ± SD | R2 | n | Release Mechanism | |
| FNEG2 | 1.874 ± 1.039 | 0.963 | 0.732 | Non Fickian Diffusion |
| FNEG4 | 1.561 ± 2.861 | 0.921 | 0.646 | Non Fickian Diffusion |
| FNEG6 | 2.571 ± 0.005 | 0.944 | 0.583 | Non Fickian Diffusion |
| Parameters | FNEG2 | FNEG4 | FNEG6 |
|---|---|---|---|
| Cmax (µg/mL) | 8.1 ± 0.13 | 8.7 ± 0.34 | 9.1 ± 0.21 |
| Tmax (h) | 12 | 12 | 12 |
| K (h−1) | 0.040 ± 0.005 | 0.036 ± 0.003 | 0.031 ± 0.002 |
| t ½ | 14.9 ± 1.98 | 15.4 ± 2.14 | 15.8 ± 1.78 |
| AUC 0–t (µg/mL.h) | 159.2 ± 18.2 | 165.5 ± 19.6 | 173.8± 21.7 |
| MRT (h) | 11.69 ± 0.26 | 11.82 ± 0.19 | 12.10 ± 0.32 |
| Parameters | Temperature | |
|---|---|---|
| 4 ± 2 °C | 40 ± 2 °C | |
| Particle size | 24.76 ± 4.12 | 24.21 ± 3.98 |
| PDI | 0.29 ± 0.35 | 0.27 ± 0.19 |
| Zeta potential (mV) | −3.81 ± 4.21 | −3.86 ± 3.29 |
| pH | 6.14 ± 0.32 | 6.31 ± 0.16 |
| Phase separation | Nil | Nil |
| Clarity | Transparent and clear | Transparent and clear |
| Drug content (%) ± SD | 97.21 ± 0.32 | 97.10 ± 0.12 |
| Color change | No change | No change |
| F. Code | Water Phase | Oil Phase | |||||
|---|---|---|---|---|---|---|---|
| Drug MTX (g) | Tween 80 (g) | PEG 400 (g) | Distilled Water | Olive Oil (g) | Clove Oil (g) | Almond Oil (g) | |
| FNE1 | 0.0 | 5 | 5 | Q.S | 7.5 | 0.0 | 0.0 |
| FNE2 | 0.25 | 5 | 5 | Q.S | 7.5 | 0.0 | 0.0 |
| FNE3 | 0.0 | 5 | 5 | Q.S | 0.0 | 7.5 | 0.0 |
| FNE4 | 0.25 | 5 | 5 | Q.S | 0.0 | 7.5 | 0.0 |
| FNE5 | 0.0 | 5 | 5 | Q.S | 0.0 | 0.0 | 7.5 |
| FNE6 | 0.25 | 5 | 5 | Q.S | 0.0 | 0.0 | 7.5 |
| F. Code | Prepared MTX Nanoemulsion | Chitosan | Triethanolamine | Distilled Water |
|---|---|---|---|---|
| FNEG2 | 50 | 1 | 1 | 48 |
| FNEG4 | 50 | 1 | 1 | 48 |
| FNEG6 | 50 | 1 | 1 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latif, M.S.; Nawaz, A.; Asmari, M.; Uddin, J.; Ullah, H.; Ahmad, S. Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery. Gels 2023, 9, 3. https://doi.org/10.3390/gels9010003
Latif MS, Nawaz A, Asmari M, Uddin J, Ullah H, Ahmad S. Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery. Gels. 2023; 9(1):3. https://doi.org/10.3390/gels9010003
Chicago/Turabian StyleLatif, Muhammad Shahid, Asif Nawaz, Mufarreh Asmari, Jalal Uddin, Hidayat Ullah, and Saeed Ahmad. 2023. "Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery" Gels 9, no. 1: 3. https://doi.org/10.3390/gels9010003
APA StyleLatif, M. S., Nawaz, A., Asmari, M., Uddin, J., Ullah, H., & Ahmad, S. (2023). Formulation Development and In Vitro/In Vivo Characterization of Methotrexate-Loaded Nanoemulsion Gel Formulations for Enhanced Topical Delivery. Gels, 9(1), 3. https://doi.org/10.3390/gels9010003