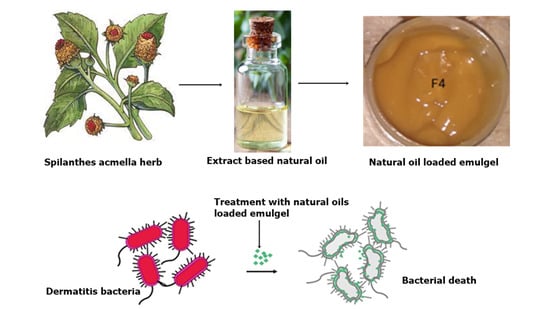
Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physical Appearance of the Emulgels
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Viscosity
2.4. Spreadability
2.5. Bio-Adhesive Strength
2.6. Extrudability
2.7. Antibacterial Studies
2.8. In Vitro Dissolution Study
2.9. Ex Vivo Permeation study
3. Conclusions
4. Materials and Methods
4.1. Materials
Method of Preparation of Spilanthes acmella Loaded Emulgel
4.2. Physical Examination
4.3. Fourier Transforms Infrared Spectroscopy (FT-IR)
4.4. Viscosity
4.5. Determination of Spreading Coefficient
4.6. Bio-Adhesive Strength Measurement
4.7. Extrudability Study of Topical Emulgel
4.8. Antibacterial Activity
4.9. In Vitro Drug Release Studies
4.10. Ex Vivo Permeation Studies through Franz’s Diffusion Cell
4.11. Statistical Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Puar, N.; Chovatiya, R.; Paller, A.S. New treatments in atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for treatment of atopic dermatitis: Current status and future prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Ollech, A.; Mashiah, J.; Lev, A.; Simon, A.J.; Somech, R.; Adam, E.; Barzilai, A.; Hagin, D.; Greenberger, S. Treatment options for DOCK8 deficiency-related severe dermatitis. J. Dermatol. 2021, 48, 1386–1393. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Silverberg, J.I.; Nosbaum, A.; Winthrop, K.L.; Guttman-Yassky, E.; Hoffmeister, K.M.; Egeberg, A.; Valdez, H.; Zhang, M.; Farooqui, S.A. Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am. J. Clin. Dermatol. 2021, 22, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Lawrence, R.; Kumar, V.; Junaid, M. Evaluation of antimicrobial activity of plant extracts on antibiotic susceptible and resistant Staphylococcus aureus strains. J. Chem. Pharm. Res. 2011, 3, 777–789. [Google Scholar]
- Papp, K.; Szepietowski, J.C.; Kircik, L.; Toth, D.; Eichenfield, L.F.; Leung, D.Y.; Forman, S.B.; Venturanza, M.E.; Sun, K.; Kuligowski, M.E. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J. Am. Acad. Dermatol. 2021, 85, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.K. The systematics of Acmella (Asteraceae-Heliantheae). In Systematic Botany Monographs; American Society of Plant Taxonomists: St. Louis, MO, USA, 1985; pp. 1–115. [Google Scholar]
- Ramsewak, R.S.; Erickson, A.J.; Nair, M.G. Bioactive N-isobutylamides from the flower buds of Spilanthes acmella. Phytochemistry 1999, 51, 729–732. [Google Scholar] [CrossRef]
- Chakraborty, A.; Devi, R.K.; Rita, S.; Sharatchandra, K.; Singh, T.I. Preliminary studies on antiinflammatory and analgesic activities of Spilanthes acmella in experimental animal models. Indian J. Pharmacol. 2004, 36, 148. [Google Scholar]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006, 6, 1–8. [Google Scholar] [CrossRef]
- Tranter, H.; Tassou, S.C.; Nychas, G. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J. Appl. Bacteriol. 1993, 74, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Newsom, M.; Bashyam, A.M.; Balogh, E.A.; Feldman, S.R.; Strowd, L.C. New and emerging systemic treatments for atopic dermatitis. Drugs 2020, 80, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An effective drug delivery system. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Azam, F.; Alqarni, M.H.; Alnasser, S.M.; Alam, P.; Jawaid, T.; Kamal, M.; Khan, S.; Alam, A. Formulation, In Vitro and In Silico Evaluations of Anise (Pimpinella anisum L.) Essential Oil Emulgel with Improved Antimicrobial Effects. Gels 2023, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- Sadeq, Z.A.; Sabri, L.A.; Al-Kinani, K.K. Natural polymer Effect on gelation and rheology of ketotifen-loaded pH-sensitive in situ ocular gel (Carbapol). J. Adv. Pharm. Educ. Res. 2022, 12, 45–50. [Google Scholar] [CrossRef]
- Hemalatha, B.; Priya, T.P.; Manasa, K.; Greeshmika, C.; Kavya, P.; Sarah, S.S.; Padmalatha, K.J.P. Optimization of Oxiconazole Topical Emulgel Formulation for the Treatment of Skin Infections. Asian J. Pharm. Technol. 2022, 1, 2. [Google Scholar] [CrossRef]
- Hasan, S.; Bhandari, S.; Sharma, A. Formulation and Evaluation of Luliconazole Emulgel. Int. J. Res. Publ. Rev. 2022, 3, 1535–1541. [Google Scholar]
- Tranchida, P.Q.; Bonaccorsi, I.; Dugo, P.; Mondello, L.; Dugo, G. Analysis of Citrus essential oils: State of the art and future perspectives. A review. Flavour Fragr. J. 2012, 27, 98–123. [Google Scholar] [CrossRef]
- Al-Qudah, T.S.; Zahra, U.; Rehman, R.; Majeed, M.I.; Sadique, S.; Nisar, S.; Tahtamouni, R.; Tahtamouni, R.W. Lemon as a source of functional and medicinal ingredient: A review. Int. J. Chem. Biochem. Sci. 2018, 14, 55–61. [Google Scholar]
- Kariman, N. Assessing comparison the effect of cooling gel pads and topical olive oil on the intensity of episiotomy pain in primiparous women. Complement. Med. J. 2015, 4, 977–986. [Google Scholar]
- Cougnard-Gregoire, A.; Merle, B.M.; Korobelnik, J.-F.; Rougier, M.-B.; Delyfer, M.-N.; Le Goff, M.; Samieri, C.; Dartigues, J.-F.; Delcourt, C. Olive oil consumption and age-related macular degeneration: The ALIENOR Study. PLoS ONE 2016, 11, e0160240. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Hombach, M.; Bloemberg, G.V.; Böttger, E.C. Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of Gram-negative bacilli. J. Antimicrob. Chemother. 2012, 67, 622–632. [Google Scholar] [CrossRef] [PubMed]
- Berger-Bächi, B.; Barberis-Maino, L.; Strässle, A.; Kayser, F.H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: Molecular cloning and characterization. Mol. Gen. Genet. MGG 1989, 219, 263–269. [Google Scholar] [CrossRef]
- Mohanty, D.; Bakshi, V.; Singh, M.A.; Aamiruddin, M.; Rashaid, M.A.; Raj, M.P.; Niharika, M.; Reddy, N.B. Formulation and Characterization of Transdermal Patches of Amlodipine Besylate Using Olive Oil as the Natural Permeation Enhancer. Am. J. Pharm. Res. 2016, 6, 5723–5729. [Google Scholar]
- Bao, Q.; Newman, B.; Wang, Y.; Choi, S.; Burgess, D.J. In vitro and ex vivo correlation of drug release from ophthalmic ointments. J. Control. Release 2018, 276, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A.; Shah, N.H.; Hussain, I.; Munawar, S.H.; Mumtaz, A.; Qureshi, N. Preparation of Spilanthes acmella based emulgel: Antimicrobial study and evaluation. Pak. J. Pharm. Sci. 2022, 35, 287–295. [Google Scholar]
- Masar, B. Formulation and Evaluation of Meloxicam as a Topical Preparation. Master’s Thesis, Collage of Pharmacy, University of Baghdad, Baghdad, Iraq, 2004. [Google Scholar]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Mohamed, M.I. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004, 6, 81–87. [Google Scholar] [CrossRef]
- Niczinger, N.; Kállai-Szabó, N.; Dredán, J.; Budai, L.; Hajdú, M.; Antal, I. Application of droplet size analysis for the determination of the required HLB of lemon oil in O/W emulsion. Curr. Pharm. Anal. 2015, 11, 11–15. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Kato, E.T.M.; de Araujo, G.L.B.; Löbenberg, R.; Monteiro, L.M.; Lourenço, F.R.; Bou-Chacra, N.A. Olive oil nanoemulsion preparation using high-pressure homogenization and d-phase emulsification—A design space approach. J. Drug Deliv. Sci. Technol. 2019, 49, 622–631. [Google Scholar] [CrossRef]
- Orafidiya, L.O.; Oladimeji, F. Determination of the required HLB values of some essential oils. Int. J. Pharm. 2002, 237, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.; Shah, S.N.H.; Iqbal, F.M. Formulation development and evaluation of diphenhydramine nasal nano-emulgel. AAPS Pharmscitech 2018, 19, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Bonacucina, G.; Cespi, M.; Palmieri, G.F. Characterization and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS Pharmscitech 2009, 10, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Dang, R.; Machale, M. Formulation and evaluation of a novel herbal gel of Stevia extract. Iran. J. Dermatol. 2009, 12, 117–122. [Google Scholar]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Tasdighi, E.; Azar, Z.J.; Mortazavi, S.A. Development and in-vitro evaluation of a contraceptive vagino-adhesive propranolol hydrochloride gel. Iran. J. Pharm. Res. IJPR 2012, 11, 13. [Google Scholar]
- Manne, N.; Yadav, H.K.; Kumar, S.H.; Khom, T.C.; Kumar, N.S. Design and evaluation of a lyophilized liposomal gel of an antiviral drug for intravaginal delivery. J. Appl. Polym. Sci. 2014, 131, 39804. [Google Scholar] [CrossRef]
- Wood, J.H.; Catacalos, G.; Lieberman, S. Adaptation of commercial viscometers for special applications in pharmaceutical rheology II: Severs extrusion rheometer. J. Pharm. Sci. 1963, 52, 375–378. [Google Scholar] [CrossRef]
- Arora, S.; Vijay, S.; Kumar, D. Phytochemical and antimicrobial studies on the leaves of Spilanthes acmella. J. Chem. Pharm. Res 2011, 3, 145–150. [Google Scholar]
- Khalil, Y.I.; Khasraghi, A.H.; Mohammed, E.J. Preparation and evaluation of physical and, rheological properties of clotrimazole emulgel. Iraqi J. Pharm. Sci. 2011, 20, 19–27. [Google Scholar] [CrossRef]
- Varma, V.N.S.K.; Maheshwari, P.; Navya, M.; Reddy, S.C.; Shivakumar, H.; Gowda, D.J. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm. J. 2014, 22, 591–599. [Google Scholar] [CrossRef]
- Chang, J.; Zhao, Y.; Zhao, W.; Venkataramanan, R.; Caritis, S.N. Obstetrical-Fetal Pharmacology Research Units Network. Quality assessment of compounded 17-hydroxyprogesterone caproate. Am. J. Obstet. Gynecol. 2014, 210, 47.e1–7. [Google Scholar] [CrossRef] [PubMed]







| Pure Extract Wave no. cm−1 | F1 Wave no. cm−1 | F2 Wave no. cm−1 | F3 Wave no. cm−1 | F4 Wave no. cm−1 |
|---|---|---|---|---|
| 3244.76 | 2918.25 | 3381.99 | 3365.43 | 3365.51 |
| 1636.32 | 2875.20 | 2957.63 | 2878.96 | 2879.54 |
| 1403.76 | 2359.77 | 2878.96 | 2133.14 | 2123.68 |
| 1048.68 | 1900.71 | 2358.40 | 1638.80 | 1638.87 |
| 930.85 | 1686.99 | 1636.40 | 1402.87 | 1559.48 |
| 677.47 | 1457.52 | 1377.52 | 1330.88 | 1403.60 |
| - | 1373.07 | 1058.74 | 1039.68 | 1332.87 |
| - | 1062.79 | 750.82 | - | 1039.43 |
| - | - | - | - | 745.53 |
| Formulation | Net wt. of Emulgel in Tube (g) Mean ± SEM | Wt. of Emulgel Extruded in (g) Mean ± SEM | Extrudability Amount in Percentage (%age) Mean ± SEM | Grade | |
|---|---|---|---|---|---|
| F1 | 12.97 ± 0.00 | 9.16 ± 0.03 | 70.67 ± 0.23 | Fair | ++ |
| F2 | 12.67 ± 0.01 | 10.86 ± 0.03 | 85.76 ± 0.19 | Good | +++ |
| F3 | 12.50 ± 0.00 | 8.6 ± 0.05 | 68.78 ± 0.41 | Fair | ++ |
| F4 | 12.15 ± 0.07 | 10.1 ± 0.05 | 83.12 ± 0.64 | Good | +++ |
| Staphylococcus aureus (Mean ± SEM) | Pseudomonas aeruginosa (Mean ± SEM) | E. coli (Mean ± SEM) | |
|---|---|---|---|
| F1 | 19.33 ± 0.28 | 0 | 23 ± 0.5 |
| F2 | 22.66 ± 0.28 | 0 | 22.33 ± 0.28 |
| F3 | 0 | 25.33 ± 0.28 | 0 |
| F4 | 25.33 ± 0.28 | 27 ± 0.5 | 27 ± 0.5 |
| Extract | 25.33 ± 0.88 | 27.33 ± 0.66 | 41.66 ± 0.88 |
| Meropenem | 0 | 17 ± 0.57 | 16.33 ± 0.33 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Formulations | 3 | 800.1 | 400.03 | 4.00 | 0.028 |
| Error | 33 | 3297.5 | 99.92 | - | - |
| Total | 35 | 4097.6 | - | - | - |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Formulations | 3 | 475.978 | 158.659 | 874.17 | 0.000 |
| Error | 8 | 1.452 | 0.181 | - | - |
| Total | 11 | 477.430 | - | - | - |
| Formulation | Zero Order | First Order | Higuchi with F0 Model | Korsmeyeres Peppas F0 Model | Hixon Crowell with tlag | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K0 | R2 | k1 | R2 | kH | R2 | kKP | R2 | n | kHC | R2 | |
| F1 | 10.45 | 0.755 | 1.90 | 0.99 | 31.94 | 0.836 | 18,222.7 | 0.908 | 0.001 | 0.085 | 0.824 |
| F2 | 13.38 | 0.971 | 0.391 | 0.99 | 37.51 | 0.987 | 58.29 | 0.988 | 0.349 | 0.081 | 0.986 |
| F3 | 15.64 | 0.987 | 0.258 | 0.995 | 43.46 | 0.994 | 38.12 | 0.994 | 0.55 | 0.099 | 0.995 |
| F4 | 13.85 | 0.913 | 0.989 | 0.983 | 39.92 | 0.954 | 33,633.64 | 0.983 | 0.001 | 0.109 | 0.952 |
| Formulation | Mean ± St. Dev. | 95% CI |
|---|---|---|
| F1 | 36.54 ± 0.193 | (36.340, 36.747) |
| F2 | 44.95 ± 0.153 | (44.7498, 45.1568) |
| F3 | 51.40 ± 0.127 | (51.1965, 51.6035) |
| F4 | 55.29 ± 0.127 | (55.0865, 55.4935) |
| Components | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Extract | 2 | 2 | 2 | 2 |
| Carbopol 935 | 1 | 2 | 1 | 2 |
| Lemon oil | 7 | 7 | - | - |
| Olive oil | - | - | 2 | 2 |
| Span 20 | 0.7 | 0.7 | 0.9 | 0.9 |
| Tween 20 | 0.2 | 0.2 | 0.08 | 0.08 |
| Liquid paraffin | 7.5 | 7.5 | 7.5 | 7.5 |
| Ethanol | 2.5 | 2.5 | 2.5 | 2.5 |
| Methyl paraben | 0.03 | 0.03 | 0.03 | 0.03 |
| Ethyl paraben | 0.01 | 0.01 | 0.01 | 0.01 |
| Propylene glycol | 5 | 5 | 5 | 5 |
| Triethanolamine | 1–2 drops | 1–2 drops | 1–2 drops | 1–2 drops |
| Water | q.s to 100 g | q.s to 100 g | q.s to 100 g | q.s to 100 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, A.; Shah, S.N.H.; Javed, H.; Mumtaz, A.; Saeed, J.; Rasheed, H.M.; Arshad, R.; Ansari, S.A.; Alkahtani, H.M.; Ansari, I.A. Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis. Gels 2023, 9, 832. https://doi.org/10.3390/gels9100832
Afzal A, Shah SNH, Javed H, Mumtaz A, Saeed J, Rasheed HM, Arshad R, Ansari SA, Alkahtani HM, Ansari IA. Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis. Gels. 2023; 9(10):832. https://doi.org/10.3390/gels9100832
Chicago/Turabian StyleAfzal, Aqsa, Syed Nisar Hussain Shah, Hina Javed, Asma Mumtaz, Javeria Saeed, Hafiz Majid Rasheed, Rabia Arshad, Siddique Akber Ansari, Hamad M. Alkahtani, and Irfan Aamer Ansari. 2023. "Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis" Gels 9, no. 10: 832. https://doi.org/10.3390/gels9100832
APA StyleAfzal, A., Shah, S. N. H., Javed, H., Mumtaz, A., Saeed, J., Rasheed, H. M., Arshad, R., Ansari, S. A., Alkahtani, H. M., & Ansari, I. A. (2023). Spilanthes acmella Extract-Based Natural Oils Loaded Emulgel for Anti-Microbial Action against Dermatitis. Gels, 9(10), 832. https://doi.org/10.3390/gels9100832