Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
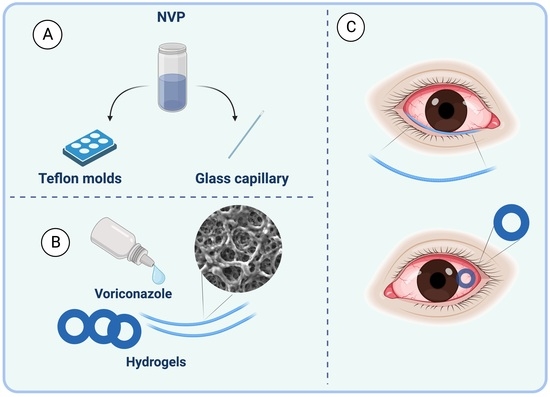
2.1. Preparation of Hydrogel Rings and Rods
2.2. Material Characterization
2.3. Drug Release
2.4. Ex-Vivo Assessment with Porcine Eyes
2.5. Cytocompatibility of Hydrogels
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Materials
4.2. Material Characterization
4.3. Drug Release In Vitro
4.4. Ex-Vivo Assessment with Porcine Eyes
4.5. HPLC Analysis
4.6. Cytocompatibility of Hydrogels
4.6.1. Cell Count Kit 8 (CCK-8)
4.6.2. Live-Dead Assay
4.6.3. Cell Proliferation in Direct Contact
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| CCK-8 | Cell Counting Kit-8 |
| DMSO | Dimethyl Sulfoxide |
| HCEC | Human Corneal Epithelial Cells |
| HPLC | High-Performance Liquid Chromatography |
| NVP | No Visible Particles |
| PBS | Phosphate-Buffered Saline |
| PEG1000 | Polyethylene Glycol 1000 |
| PLGA | Polylactic-co-glycolic acid |
| PTFE | Polytetrafluoroethylene |
| PCR | Polymerase Chain Reaction |
| SEM | Scanning Electron Microscope |
| SEM-EDX | Scanning Electron Microscope with Energy Dispersive X-ray Spectroscopy |
| UV-Vis | Ultraviolet-Visible Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Niu, L.; Liu, X.; Ma, Z.; Yin, Y.; Sun, L.; Yang, L.; Zheng, Y. Fungal keratitis: Pathogenesis, diagnosis and prevention. Microb. Pathog. 2020, 138, 103802. [Google Scholar] [CrossRef] [PubMed]
- Badian, R.A.; Ekman, L.; Pripp, A.H.; Utheim, T.P.; Englund, E.; Dahlin, L.B.; Rolandsson, O.; Lagali, N. Comparison of Novel Wide-Field In Vivo Corneal Confocal Microscopy with Skin Biopsy for Assessing Peripheral Neuropathy in Type 2 Diabetes. Diabetes 2023, 72, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.W.Y.M.; Harrison, R.B.; Hau, S.B.; Alexander, C.L.P.; Tole, D.M.F.; Avadhanam, V.S.M. Comparison of In Vivo Confocal Microscopy, PCR and Culture of Corneal Scrapes in the Diagnosis of Acanthamoeba Keratitis. Cornea 2017, 37, 480–485. [Google Scholar] [CrossRef]
- Watson, S.L.; Cabrera-Aguas, M.; Keay, L.; Khoo, P.; McCall, D.; Lahra, M.M. The clinical and microbiological features and outcomes of fungal keratitis over 9 years in Sydney, Australia. Mycoses 2020, 63, 43–51. [Google Scholar] [CrossRef]
- Hoffman, J.J.; Arunga, S.; Ahmed, A.H.A.M.; Hu, V.H.; Burton, M.J. Management of Filamentous Fungal Keratitis: A Pragmatic Approach. J. Fungi 2022, 8, 1067. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Sitnova, A.; Svetozarskiy, S. Modern Technologies in Diagnosis of Fungal Keratitis (Review). Sovrem. Tehnol. Med. 2023, 15, 73–84. [Google Scholar] [CrossRef]
- Reginatto, P.; Agostinetto, G.d.J.; Fuentefria, R.D.N.; Marinho, D.R.; Pizzol, M.D.; Fuentefria, A.M. Eye fungal infections: A mini review. Arch. Microbiol. 2023, 205, 1–26. [Google Scholar] [CrossRef]
- Sarmout, M.; Xiao, Y.; Hu, X.; Rakhmetova, A.; Koole, L.H. A novel approach to achieve semi-sustained drug delivery to the eye through asymmetric loading of soft contact lenses. Heliyon 2023, 9, e16916. [Google Scholar] [CrossRef]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Mazet, R.; Yameogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Geze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.Y.; Abdulrahman, N.S.; Schatzlein, A.G.; Uchegbu, I.F. A polymeric aqueous tacrolimus formulation for topical ocular delivery. Int. J. Pharm. 2021, 599, 120364. [Google Scholar] [CrossRef] [PubMed]
- Uwaezuoke, O.; Du Toit, L.C.; Kumar, P.; Ally, N.; Choonara, Y.E. Linoleic Acid-Based Transferosomes for Topical Ocular Delivery of Cyclosporine A. Pharmaceutics 2022, 14, 1695. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Yang, X.; Chen, S.; Wang, Q.; Zhang, A.; Tang, B. Cyclodextrin-based host–guest supramolecular hydrogels for local drug delivery. Coord. Chem. Rev. 2021, 454, 214352. [Google Scholar] [CrossRef]
- Lynch, C.R.; Kondiah, P.P.D.; Choonara, Y.E.; du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel Biomaterials for Application in Ocular Drug Delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive manufacturing of sustainable biomaterials for biomedical applications. Asian J. Pharm. Sci. 2023, 18, 100812. [Google Scholar] [CrossRef]
- Cuming, R.S.; Abarca, E.M.; Duran, S.; Wooldridge, A.A.; Stewart, A.J.; Ravis, W.; Babu, R.J.; Lin, Y.-J.; Hathcock, T. Development of a Sustained-Release Voriconazole-Containing Thermogel for Subconjunctival Injection in Horses. Investig. Opthalmol. Vis. Sci. 2017, 58, 2746. [Google Scholar] [CrossRef]
- Okur, N.U.; Yozgatli, V.; Senyigit, Z. Formulation and detailed characterization of voriconazole loaded in situ gels for ocular application. Ank. Univ. Eczaci. Fak. Derg. 2020, 44, 33–49. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.J.; Jeong, B. Hyaluronic acid-g-PPG and PEG-PPG-PEG hybrid thermogel for prolonged gel stability and sustained drug release. Carbohydr. Polym. 2022, 291, 119559. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.J.; Lai, J.Y. Thermogels containing sulfated hyaluronan as novel topical therapeutics for treatment of ocular surface inflammation. Mater. Today Bio 2021, 13, 100183. [Google Scholar] [CrossRef]
- Mora-Pereira, M.; Abarca, E.M.; Duran, S.; Ravis, W.; McMullen, R.J.; Fischer, B.M.; Lee, Y.-H.P.; Wooldridge, A.A. Sustained-release voriconazole-thermogel for subconjunctival injection in horses: Ocular toxicity and in-vivo studies. BMC Vet. Res. 2020, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Vivero-Lopez, M.; Concheiro, A. Contact lenses that transform gold into nanoparticles for prophylaxis of light-related events and photothermal therapy. Int. J. Pharm. 2023, 641, 123048. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Oom, M.S.; Alvarez-Lorenzo, C.; Saramago, B.; Serro, A.P. Moxifloxacin-imprinted silicone-based hydrogels as contact lens materials for extended drug release. Eur. J. Pharm. Sci. 2021, 156, 105591. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Kanani, P.A.; Jadav, H.J.; Desai, B.V.; Desai, D.T.; Patel, H.P.; Shetty, K.H.; Shah, D.O.; Willcox, M.D. Timolol-eluting graphene oxide laden silicone contact lens: Control release profile with improved critical lens properties. J. Drug Deliv. Sci. Technol. 2022, 69, 103134. [Google Scholar] [CrossRef]
- Al-Kinani, A.A.; Zidan, G.; Elsaid, N.; Seyfoddin, A.; Alani, A.W.G.; Alany, R.G. Ophthalmic gels: Past, present and future. Adv. Drug Deliv. Rev. 2018, 126, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Pijls, R.T.; Cruysberg, L.P.; Nuijts, R.M.M.A.; Dias, A.A.; Koole, L.H. Capacity and tolerance of a new device for ocular drug delivery. Int. J. Pharm. 2007, 341, 152–161. [Google Scholar] [CrossRef]
- Noroozi, R.; Arif, Z.U.; Taghvaei, H.; Khalid, M.Y.; Sahbafar, H.; Hadi, A.; Sadeghianmaryan, A.; Chen, X. 3D and 4D Bioprinting Technologies: A Game Changer for the Biomedical Sector? Ann. Biomed. Eng. 2023, 51, 1683–1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Koole, L.H.; Gao, C.; Yang, D.; Yang, L.; Zhang, C.; Li, H. The potential utility of hybrid photo-crosslinked hydrogels with non-immunogenic component for cartilage repair. NPJ Regen. Med. 2021, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef]
- Tijink, M.; Janssen, J.; Timmer, M.; Austen, J.; Aldenhoff, Y.; Kooman, J.; Koole, L.; Damoiseaux, J.; van Oerle, R.; Henskens, Y.; et al. Development of novel membranes for blood purification therapies based on copolymers of N-vinylpyrrolidone and n-butylmethacrylate. J. Mater. Chem. B 2013, 1, 6066. [Google Scholar] [CrossRef]
- Ladetto, M.F.; Lázaro-Martínez, J.M.; Devoto, T.B.; Briceño, V.J.; Castro, G.R.; Cuestas, M.L. Quantitative determination of voriconazole by thionine reduction and its potential application in a pharmaceutical and clinical setting. Anal. Methods 2023, 15, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Pijls, R.T.; Sonderkamp, T.; Daube, G.W.; Krebber, R.; Hanssen, H.H.; Nuijts, R.M.M.A.; Koole, L.H. Studies on a new device for drug delivery to the eye. Eur. J. Pharm. Biopharm. 2005, 59, 283–288. [Google Scholar] [CrossRef]
- Datta, D.; Roy, G.; Garg, P.; Venuganti, V.V.K. Ocular delivery of cyclosporine A using dissolvable microneedle contact lens. J. Drug Deliv. Sci. Technol. 2022, 70, 103211. [Google Scholar] [CrossRef]
- Morgan, S.R.; Pilia, N.; Hewitt, M.; Moses, R.L.; Moseley, R.; Lewis, P.N.; Morrison, P.W.; Kelly, S.L.; Parker, J.E.; Whitaker, D.; et al. Controlled in vitro delivery of voriconazole and diclofenac to the cornea using contact lenses for the treatment of Acanthamoeba keratitis. Int. J. Pharm. 2020, 579, 119102. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-Osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.; Gober, M.D.; Bashir, H.; O’Day, C.; Blair, I.A.; Mesaros, C.; Weng, L.; Huang, A.; Chen, A.; Tang, R.; et al. Voriconazole enhances UV-induced DNA damage by inhibiting catalase and promoting oxidative stress. Exp. Dermatol. 2020, 29, 29–38. [Google Scholar] [CrossRef]
- Martos, A.I.; Martin-Mazuelos, E.; Romero, A.; Serrano, C.; Gonzalez, T.; Almeida, C.; Puche, B.; Canton, E.; Peman, J.; Espinel-Ingroff, A. Evaluation of disk diffusion method compared to broth microdilution for antifungal susceptibility testing of 3 echinocandins against Aspergillus spp. Diagn. Microbiol. Infect. Dis. 2012, 73, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.G.; Morrison, P.W.J.; Boostrom, H.M.; Morgan, S.R.; Fallon, M.; Lewis, P.N.; Whitaker, D.; Brancale, A.; Varricchio, C.; Quantock, A.J.; et al. In Vitro Topical Delivery of Chlorhexidine to the Cornea: Enhancement Using Drug-Loaded Contact Lenses and β-Cyclodextrin Complexation, and the Importance of Simulating Tear Irrigation. Mol. Pharm. 2020, 17, 1428–1441. [Google Scholar] [CrossRef]
- Salètes, M.; Vartin, M.; Mocquot, C.; Chevalier, C.; Grosgogeat, B.; Colon, P.; Attik, N. Mesoporous Bioactive Glasses Cytocompatibility Assessment: A Review of In Vitro Studies. Biomimetics 2021, 6, 9. [Google Scholar] [CrossRef]
- Lee, C.; O’Connell, C.D.; Onofrillo, C.; Choong, P.F.M.; Di Bella, C.; Duchi, S. Human articular cartilage repair: Sources and detection of cytotoxicity and genotoxicity in photo-crosslinkable hydrogel bioscaffolds. Stem Cells Transl. Med. 2020, 9, 302–315. [Google Scholar] [CrossRef]






| Hydrogel Rings (after 2 h), µg | ||
| Sample | Dry form | Wet form |
| 1 | 40.232 | 55.92 |
| 2 | 36.296 | 57.43 |
| 3 | 44.716 | 66.406 |
| 4 | 43.748 | 56.034 |
| 5 | 36.512 | 52.828 |
| 6 | 48.324 | 64.72 |
| 7 | 45.084 | 59.43 |
| 8 | 48.522 | 69.734 |
| Mean ± SD | 42.93 ± 4.8 | 60.31 ± 6.0 |
| Bioavailability | 25.1 ± 2.5% | 35.8 ± 3.2% |
| 1% Voriconazole eye drops (after 2 h), µg [34] | ||
| Drug amount | Bioavailability | |
| Mean ± SD | 6 ± 1.5 | 3.9 ± 1.0% |
| Mold # | Outer Diameter, mm | Inner Diameter, mm | Depth, mm |
|---|---|---|---|
| 1 | 10.0 | 6.0 | 1.0 |
| 2 | 10.0 | 6.0 | 0.8 |
| 3 | 9.0 | 5.0 | 1.0 |
| 4 | 9.0 | 5.0 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhmetova, A.; Yi, Z.; Sarmout, M.; Koole, L.H. Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels 2023, 9, 933. https://doi.org/10.3390/gels9120933
Rakhmetova A, Yi Z, Sarmout M, Koole LH. Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels. 2023; 9(12):933. https://doi.org/10.3390/gels9120933
Chicago/Turabian StyleRakhmetova, Aiym, Zhiqi Yi, Malake Sarmout, and Leo H. Koole. 2023. "Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery" Gels 9, no. 12: 933. https://doi.org/10.3390/gels9120933
APA StyleRakhmetova, A., Yi, Z., Sarmout, M., & Koole, L. H. (2023). Sustained Release of Voriconazole Using 3D-Crosslinked Hydrogel Rings and Rods for Use in Corneal Drug Delivery. Gels, 9(12), 933. https://doi.org/10.3390/gels9120933