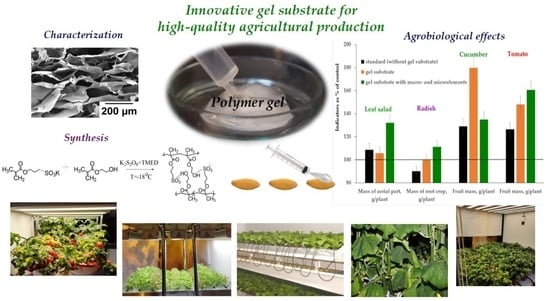
Polymer Gel Substrate: Synthesis and Application in the Intensive Light Artificial Culture of Agricultural Plants
Abstract
:1. Introduction
- (1)
- to synthesize gel substrates based on copolymers of potassium 3-sulfopropyl methacrylate and 2-hydroxyethyl methacrylate with and without the addition of macro- and microelements;
- (2)
- to study under controlled conditions the effect of the created gel substrates on the physiological state of plants (lettuce, radish, cucumber and tomato), namely the content of photosynthetic pigments, the activities of antioxidant enzymes, the intensity of lipid peroxidation, the elemental composition in plant leaves during the vegetative period (lettuce, radish, cucumber and tomato) and generative period of their development (tomato, and cucumber), and plants’ growth and productivity.
2. Results and Discussion
2.1. Synthesis of Gel Substrates
2.2. The Influence of the Created Gel Substrates on the Physiological State, Growth and Productivity of Plants
2.2.1. Physiological State of Plants
- -
- -
- -
- The content of macro- and microelements in plant leaves at the early stages of their development (Table 3), making it possible to judge the situation with the vital supply of plant micronutrients necessary for growth and development, which are also cofactors of enzymes and constituent elements of pigments and other substances involved in the physiological and metabolic processes of the plant organism [32].
- -
- An increase relative to the control values of the content of chlorophyll a and the total chlorophylls in the leaves of plants in variants of root-inhabited environment with TAS + GG, TAS + mGG for lettuce—by 21–23% and by 10–12%, respectively; for radishes—by 4–5% and by 4–6%; for cucumber—by 7–33% and 5–33%; for tomato—by 18–23% and 21–32% (Table 1). Moreover, the values of these indicators were also in the form of a trend or significantly higher than those in the reference variant when growing plants on root-inhabited environment with TAS + SG in lettuce, radish and tomato plants, and in cucumber plants—only in the variant with root-inhabited environment TAS + GG.
- -
- A significant increase in the chlorophyll b contents values in leaves in variants of root-inhabited environment with TAS + GG, and TAS + mGG relative to the control: in tomato—34 and 62%; in cucumber—significant only in the variant with root-inhabited environment TAS + GG by 33%. For radishes, there are no significant changes; for lettuce, there is a significant or trending decrease in the content of this indicator by 16 and 30% (Table 1). Moreover, the values of this indicator were in the form of a trend or significantly higher than those in the reference variant in the TAS + GG and TAS + mGG variants for radish, in the TAS + GG variant for cucumber, in the TAS + mGG variant for tomato. Lower values were observed in the TAS + mGG variant for lettuce and cucumber, and in the TAS + GG variant for tomato.
- -
- Decreased relative to control values of carotenoid content in plant leaves in variants of root-inhabited environment with TAS + GG or with TAS + mGG in lettuce by 12–26%; in cucumber—only in the TAS + mGG variant by 11% (Table 1). Moreover, compared to the reference variant, which had significantly lower values relative to the control, the variants with gel substrates promote a decrease in its negative effects in lettuce plants, neutralize it for radish, and did not differ in indicators for tomato plants, and had lower values in the TAS + mGG variant by cucumber plants.
2.2.2. Plant Growth and Productivity Indicators of Plant
2.2.3. Quality and Safety Indicators of Plant Production
3. Conclusions
4. Materials and Methods
4.1. Materials
4.1.1. Chemical Materials
4.1.2. Biological Materials
4.2. Methods
4.2.1. Synthesis of Gels
4.2.2. ATR-FTIR and 13C NMR
4.2.3. Scanning Electron Microscope
4.2.4. Measurement of Specific Surface Area, Pore and Porosity Analysis
4.2.5. Biological Experiment Design and Conditions
Experimental Design
- Evaluation of the influence of the hydrogels introduction to the leaf salad seed on the plants physiological state, biochemical composition, growth, quality of the obtained plant production;
- Study of the influence of the hydrogels introduction to the radish plants seed on the plants physiological state, biochemical composition, growth, productivity and quality of the obtained plant production;
- Study of the influence of the hydrogels introduction to the tomato plants seed on the plants physiological state, biochemical composition, growth, productivity and quality of the obtained plant production;
- Evaluation of the influence of the hydrogels introduction to the cucumber plants seed on the plants physiological state, biochemical composition, growth, productivity and quality of the obtained plant production.
- TAS—thin-layer analogue of soil—control;
- TAS + CS (standard)—thin-layer analogue of soil + clay [40] suspension (layer 1 mm thick; cation exchange capacity of clay 22.1 mg-eq per 100 g);
- TAS + GG dilution 1:500—thin-layer analogue of soil + hydrogel (layer 1 mm thick);
- TAS + mGG dilution 1:500—thin-layer analogue of soil + hydrogel modified with the addition of macro- and microelements (layer 1 mm thick).
- The density of the leaf lettuce cenosis was formed based on 1 m2 of usable area of the growing light installation: 40 lettuce plants. The duration of each experiment: 28 days from sowing the seeds to plants aerial part harvesting.
- Replications per variant by radish—28 plants. The duration of each experiment was 26 days from seed sowing to root vegetable harvesting.
- Replications per variant by tomato—10 plants. The duration of each experiment was 90 days from seed sowing to fruit harvesting.
- Replications per variant by cucumber—10 plants. We formed their plants into one stem with a length of 2 m. The duration of each experiment was 58 days from seed sowing to fruit harvesting.
- The content of photosynthetic pigments;
- The activity of antioxidant enzymes;
- The content of macro- and microelements;
- The biometric indicators of plant growth: plant leaf area, number of leaves, cross-sectional area of the stem (tomatoes, and cucumbers), length and diameter of root crops (radish), and fresh and dry weight of plant organs (stems, leaves, roots, and % dry substances).
4.2.6. Plant Analyses
Morphology Measurements
Photosynthetic Pigments Analysis
Antioxidant System Activity
Plant Production’s Quality and Safety Indicators
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demitri, C.; Scalera, F.; Madaghiele, M.; Sannino, A.; Maffezzoli, A. Potential of cellulose-based superabsorbent hydrogels as water reservoir in agriculture. Int. J. Polym. Sci. 2013, 2013, 435073. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Essawy, H.A.; Ghazy, M.B.M.; El-Hai, F.A.; Mohamed, M.F. Superabsorbent hydrogels via graft polymerization of acrylic acid from chitosan-cellulose hybrid and their potential in controlled release of soil nutrients. Int. J. Biol. Macromol. 2016, 89, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B.; Basak, B.B.; Sarkar, S.; Mandal, S. Adaptive Soil Management: From Theory to Practices; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- López-Velázquez, J.C.; Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Qui-Zapata, J.A.; García-Morales, S.; Navarro-López, D.E.; Luna-Bárcenas, G.; Vassallo-Brigneti, E.C.; García-Carvajal, Z.Y. Gelatin–chitosan–PVA hydrogels and their application in agriculture. J. Chem. Technol. Biotechnol. 2019, 94, 3495–3504. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.; Zhang, P.; Zhou, J.; Lu, X.; Tian, W. Carbohydrate polymers exhibit great potential as effective elicitors in organic agriculture: A review. Carbohydr. Polym. 2020, 230, 115637. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. Applications of absorbent polymers for sustainable plant protection and crop yield. Sustainability 2021, 13, 3253. [Google Scholar] [CrossRef]
- Sikder, A.; Pearce, A.K.; Parkinson, S.J.; Napier, R.; O’Reilly, R.K. Recent trends in advanced polymer materials in agriculture related applications. ACS Appl. Polym. Mater. 2021, 3, 1203–1217. [Google Scholar] [CrossRef]
- Berninger, T.; Dietz, N.; Gonzále Lópezz, Ó. Water-soluble polymers in agriculture: Xanthan gum as eco-friendly alternative to synthetics. Microb. Biotechnol. 2021, 14, 1881–1896. [Google Scholar] [CrossRef]
- Chang, L.; Xu, L.; Liu, Y.; Qiu, D. Superabsorbent polymers used for agricultural water retention. Polym. Test. 2021, 94, 115637. [Google Scholar] [CrossRef]
- Grabowska-Polanowska, B.; Garbowski, T.; Bar-Michalczyk, D.; Kowalczyk, A. The benefits of synthetic or natural hydrogels application in agriculture: An overview article. J. Water Land Dev. 2021, 51, 208–224. [Google Scholar]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current trends and challenges in the synthesis and applications of chitosan-based nanocomposites for plants: A review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef] [PubMed]
- Sousa, H.R.; Lima, I.S.; Neris, L.M.L.; Silva, A.S.; Nascimento, A.M.S.S.; Araújo, F.P.; Ratke, R.F.; Silva, D.A.; Osajima, J.A.; Bezerra, L.R.; et al. Superabsorbent Hydrogels Based to Polyacrylamide/Cashew Tree Gum for the Controlled Release of Water and Plant Nutrients. Molecules 2021, 26, 2680. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Chen, H.; Cheng, D. Environmentally friendly hydrogel: A review of classification, preparation and application in agriculture. Sci. Total Environ. 2022, 846, 157303. [Google Scholar] [CrossRef] [PubMed]
- Krasnopeeva, E.L.; Panova, G.G.; Yakimansky, A.V. Agricultural applications of superabsorbent polymer hydrogels. Int. J. Mol. Sci. 2022, 23, 15134. [Google Scholar] [CrossRef] [PubMed]
- Malka, E.; Margel, S. Engineering of PVA/PVP Hydrogels for Agricultural Applications. Gels 2023, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Lisuzzo, L.; Lazzara, G.; Milioto, S. Printable Hydrogels Based on Alginate and Halloysite Nanotubes. Int. J. Mol. Sci. 2022, 23, 3294. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Kuznetsov, V.A.; Lavlinskaya, M.S.; Ostankova, I.V.; Selemenev, V.F.; Semenov, V.N.; Loukine, A.L. Watersorption ability of rare cross-linked polymeric material with superabsorbent properties. Sorbtsionnye I Khromatograficheskie Protsessy 2017, 17, 484–489. [Google Scholar]
- Asamatdinov, A. New water-keeping soil additives. Mod. Chem. Appl. 2018, 6, 1000246. [Google Scholar] [CrossRef]
- Khodadadi Dehkordi, D.; Shamsnia, S.A. Application of reclaimed sodium polyacrylate to increase soil water retention. Clean Soil Air Water 2020, 48, 2000068. [Google Scholar] [CrossRef]
- Laman, N.A.; Vavilova, T.V.; Sudnik, A.F. The use of polymeric materials for structuring soils and greenhouse substrates (review). Bot. (Res.) Collect. Sci. Pap. 2010, 39, 332–349. [Google Scholar]
- Dehkordi, D.K. Effect of superabsorbent polymer on soil and plants on steep surfaces. Water Environ. J. 2018, 32, 158–163. [Google Scholar] [CrossRef]
- Ermakov, E.I. Selected Works; PNPI RAS Publishing: St. Petersburg, Russia, 2009; 192p. (In Russian) [Google Scholar]
- Panova, G.G.; Udalova, O.R.; Kanash, E.V.; Galushko, A.S.; Kochetov, A.A.; Priyatkin, N.S.; Arkhypov, M.V.; Chernousov, I.N. Fundamentals of physical modeling of “ideal” agroecosystems. Tech. Phys. 2020, 65, 1563–1569. [Google Scholar] [CrossRef]
- Sinyavina, N.G.; Kochetov, A.A.; Kocherina, N.V.; Egorova, K.V.; Kurina, A.B.; Panova, G.G.; Chesnokov, Y.V. Breeding Approaches for Controlled Conditions of Artificial Light Culture for Small Radish and Radish (Raphanus sativus L.). Horticulturae 2023, 9, 678. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Wang, Y.; Hu, Z.; Wang, R. Characterization of slow-release collagen-g-poly(acrylic acid-co-2-acrylamido-2-methyl-1-propane sulfonic acid)–iron(III) superabsorbent polymer containing fertilizer. J. Appl. Polym. Sci. 2019, 136, 47178. [Google Scholar] [CrossRef]
- Gala, S.; Mao, Y.; Bao, X.; Ma, B.; Pan, Y.; Huang, J. Preparation of high water-absorbing hydrogel based on grafted micro/nano cellulose. Bioresourses 2021, 16, 1256–1275. [Google Scholar] [CrossRef]
- Laishevkina, S.; Kuleshova, T.; Panova, G.; Ivan’kova, E.; Iakobson, O.; Dobrodumov, A.; Shevchenko, N.; Yakimansky, A. Sulfonic Cryogels as innovative materials for biotechnological applications: Synthesis, modification, and biological activity. Int. J. Mol. Sci. 2023, 24, 2949. [Google Scholar] [CrossRef]
- Kanash, E.V.; Sinyavina, N.G.; Rusakov, D.V.; Egorova, K.V.; Panova, G.G.; Chesnokov, Y.V. Morpho-Physiological, Chlorophyll Fluorecence and Diffuse Reflectance Spectra Characteristics of Lettuce under the Main Macronutrient Deficiency. Horticulturae 2023, 9, 1185. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The Significance of Reactive Oxygen Species and Antioxidant Defense System in Plants: A Concise Overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Tariq, A.; Zeng, F.; Graciano, C.; Ullah, A.; Sadia, S.; Ahmed, Z.; Murtaza, G.; Ismoilov, K.; Zhang, Z. Regulation of Metabolites by Nutrients in Plants. In Plant Ionomics: Sensing, Signaling, and Regulation; Singh, V.P., Siddiqui, M.H., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Nichiporovich, A.A. Photosynthesis and the Productive Process; M Science: New York, NY, USA, 1988; 276p. (In Russian) [Google Scholar]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effect of organic and conventional cropping systems on ascorbic acid, vitamin c, flavonoids, nitrate, and oxalate in 27 varieties of spinach (Spinacia oleracea L.). J. Agric. Food Chem. 2012, 60, 3144–3150. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Zhang, X.; You, S.; Tian, Y.; Li, J. Comparison of plastic film, biodegradable paper and bio-based film mulching for summer tomato production: Soil properties, plant growth, fruit yield and fruit quality. Sci. Hortic. 2019, 249, 38–48. [Google Scholar] [CrossRef]
- Panova, G.G.; Semenov, K.N.; Zhuravleva, A.S.; Khomyakov, Y.V.; Volkova, E.N.; Mirskaya, G.V.; Artemyeva, A.M.; Iamalova, N.R.; Dubovitskaya, V.I.; Udalova, O.R. Obtaining Vegetable Production Enriched with Minor Micronutrients Using Fullerene Derivatives. Horticulturae 2023, 9, 828. [Google Scholar] [CrossRef]
- Panova, G.G.; Chernousov, I.N.; Udalova, O.R.; Alexandrov, A.V.; Karmanov, I.V.; Anikina, L.M.; Sudakov, V.L.; Yakushev, V.P. Scientific basis for large year-round yields of high-quality crop products under artificial lighting. Russ. Agric. Sci. 2015, 41, 335–339. [Google Scholar] [CrossRef]
- Gilinskaya, L.G.; Grigorieva, T.N.; Razvorotneva, L.I.; Trofimova, L.B. Composition and Physicochemical Properties of Natural Blue Clays. Chem. Sustain. Dev. 2008, 16, 143–153. [Google Scholar]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Kumar, G.N.M.; Knowles, N.R. Changes in lipid peroxidation and lipolytic and free radical scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed-tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef]
- Lukatkin, A.S. Contribution of oxidative stress to development of cold-induced damage on leaves of chilling-sensitive plants: 1. Reactive oxygen species formation during plant during plant chilling. Russ. J. Plant Physiol. 2002, 49, 622–627. [Google Scholar] [CrossRef]
- Pochinok, H.N. Methods of Biochemical Analysis of Plants; Naukovo Dumka: Kyiv, Ukraine, 1976. (In Russian) [Google Scholar]
- State Standard of Russian Federation 24556-89; Products of Fruits and Vegetables. Processing. Methods for Determination of Vitamin C. Standartinform: Moscow, Russia, 2003. (In Russian)
- MU N 5048-89; Guidelines for the Determination of Nitrates and Nitrites in Crop Production. USSR Ministry of Health, USSR State Agroprom: Moscow, Russia, 1989; 52p. (In Russian)
- Interstate Standard GOST EN 14084—2014; Foodstuffs. Definition of Trace Elements. Determination of Lead, Cadmium, Zinc, Copper and Iron Content by Atomic Absorption Spectrometry (AAS) after Microwave Digestion. Eurasian Council for Standardization, Metrology and Certification: Minsk, Belarus, 2014; 17p. (In Russian)
- echnical Regulation of the Customs Union “On Food Safety” (TR TS 021/2011) dated 09.12.2011 N021/2011 “(as Amended on 14 July 2021), Official Website of the Customs Union Commission. 173p. Available online: www.tsouz.ru (accessed on 15 December 2021).
- Assessment of the quality of food products and assessment of the population’s access to domestic food products, contributing to the elimination of macro- and micronutrient deficiencies: Guidelines. MP 2.3.7.0168-20. Bull. Regul. Methodol. Doc. State Sanit. Epidemiol. Superv. Russ. Fed. 2020, 2, 43–106. (In Russian)





| Indicators | Leaves | |||
|---|---|---|---|---|
| Root-Inhabited Environment * | ||||
| TAS (Control) | TAS + SC (Etalon) | TAS + GG | TAS + mGG | |
| Leaf salad cv. Typhoon | ||||
| Chlorophyll a, µg 100 g−1 FM | 70.57 ± 3.53 b | 80.24 ± 4.01 a | 85.56 ± 4.28 a | 87.02 ± 4.35 a |
| Chlorophyll b, µg 100 g−1 FM | 22.64 ± 1.13 a | 18.59 ± 0.93 b | 19.11 ± 0.96 b | 15.97 ± 0.80 c |
| Total chlorophyll, µg 100 g−1 FM | 93.21 ± 4.66 b | 98.83 ± 4.94 ab | 104.67 ± 5.23 a | 102.99 ± 5.15 ab |
| Carotenoid, µg 100 g−1 FM | 25.47 ± 1.27 a | 15.98 ± 0.80 d | 22.51 ± 1.13 b | 18.88 ± 0.94 c |
| Radish cv. Sacharok | ||||
| Chlorophyll a, µg 100 g−1 FM | 70.83 ± 3.54 a | 68.49 ± 3.42 a | 73.72 ± 3.69 a | 74.39 ± 3.72 a |
| Chlorophyll b, µg 100 g−1 FM | 23.07 ± 1.15 ab | 22.06 ± 1.10 b | 24.17 ± 1.21 ab | 24.84 ± 1.24 a |
| Total chlorophyll, µg 100 g−1 FM | 93.91 ± 4.70 a | 90.55 ± 4.53 a | 97.89 ± 4.89 a | 99.22 ± 4.96 a |
| Carotenoid, µg 100 g−1 FM | 23.24 ± 1.16 a | 20.60 ± 1.03 b | 23.11 ± 1.16 a | 24.25 ± 1.21 a |
| Cucumber hybrid F1 Neva | ||||
| Chlorophyll a, µg 100 g−1 FM | 116.96 ± 5.85 b | 125.42 ± 6.27 b | 156.01 ± 7.80 a | 124.75 ± 6.24 b |
| Chlorophyll b, µg 100 g−1 FM | 34.57 ± 1.73 b | 36.89 ± 1.84 b | 46.03 ± 2.30 a | 35.17 ± 1.76 b |
| Total chlorophyll, µg 100 g−1 FM | 151.52 ± 7.58 b | 162.30 ± 8.12 b | 202.05 ± 10.10 a | 159.83 ± 7.99 b |
| Carotenoid, µg 100 g−1 FM | 40.44 ± 2.02 a | 43.94 ± 2.20 a | 43.34 ± 2.17 a | 35.98 ± 1.80 b |
| Tomato cv. Natasha | ||||
| Chlorophyll a, µg 100 g−1 FM | 99.00 ± 4.95 b | 111.39 ± 5.57 a | 116.37 ± 5.82 a | 121.76 ± 6.09 a |
| Chlorophyll b, µg 100 g−1 FM | 30.02 ± 1.50 d | 44.70 ± 2.24 b | 40.27 ± 2.01 c | 48.61 ± 2.43 a |
| Total chlorophyll, µg 100 g−1 FM | 129.02 ± 6.45 b | 156.09 ± 7.80 a | 156.64 ± 7.83 a | 170.37 ± 8.52 a |
| Carotenoid, µg 100 g−1 FM | 40.30 ± 2.02 a | 43.17 ± 2.16 a | 41.69 ± 2.08 a | 40.57 ± 2.03 a |
| Indicators | Leaves | |||
|---|---|---|---|---|
| Root-Inhabited Environment * | ||||
| TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | |
| Leaf salad cv. Typhoon | ||||
| CAT, µM H2O2 mg−1 Protein min−1 | 237.39 ± 11.87 ab | 237.06 ± 11.85 ab | 215.54 ± 10.78 b | 250.84 ± 12.54 a |
| LPO, µM g−1 | 0.0026 ± 0.0001 a | 0.0024 ± 0.0001 ab | 0.0023 ± 0.0001 b | 0.0022 ± 0.0001 b |
| Radish cv. Sacharok | ||||
| POX, U s−1 g−1 | 2.08 ± 0.10 a | 2.20 ± 0.11 a | 2.09 ± 0.10 a | 2.27 ± 0.11 a |
| CAT, µM H2O2 mg−1 Protein min−1 | 325.93 ± 16.30 a | 297.61 ± 14.89 a | 323.51 ± 16.18 a | 318.82 ± 15.94 a |
| LPO, µM g−1 | 0.0072 ± 0.0004 a | 0.0066 ± 0.0003 b | 0.0049 ± 0.0002 d | 0.0060 ± 0.0003 c |
| Cucumber hybrid F1 Neva | ||||
| POX, U s−1 g−1 | 8.23 ± 0.41 bc | 7.86 ± 0.39 c | 9.11 ± 0.46 b | 15.87 ± 0.79 a |
| CAT, µM H2O2 mg−1 Protein min−1 | 443.42 ± 22.17 a | 432.53 ± 21.63 a | 406.84 ± 20.34 a | 404.6 ± 20.23 a |
| LPO, µM g−1 | 0.0041 ± 0.0002 c | 0.0046 ± 0.0003 b | 0.0051 ± 0.0003 a | 0.0050 ± 0.0003 ab |
| Tomato cv. Natasha | ||||
| POX, U s−1 g−1 | 17.02 ± 0.85 a | 16.98 ± 0.85 a | 15.96 ± 0.80 ab | 15.02 ± 0.75 b |
| CAT, µM H2O2 mg−1 Protein min−1 | 92.0 ± 4.60 a | 100.87 ± 5.04 a | 83.09 ± 4.15 b | 98.35 ± 4.92 a |
| LPO, µM g−1 | 0.0125 ± 0.0006 a | 0.0109 ± 0.0005 b | 0.0114 ± 0.0006 b | 0.0107 ± 0.0005 b |
| Indicators | Leaf Salad cv. Typhoon | Radish cv. Sacharok | Cucumber Hybrid F1 Neva | Tomato cv. Natasha | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root-Inhabited Environment * | ||||||||||||||||
| TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
| Raw ash, % a.d.m. | 20.78 ± 1.04 a | 21.5 ± 1.08 a | 20.60 ± 1.03 a | 19.71 ± 0.99 a | 24.64 ± 1.23 a | 24.53 ± 1.23 a | 24.65 ± 1.23 a | 24.94 ± 1.25 a | 14.46 ± 0.72 a | 15.34 ± 0.77 a | 15.73 ± 0.79 a | 15.60 ± 0.78 a | 14.52 ± 0.73 b | 16.64 ± 0.83 a | 15.75 ± 0.79 ab | 15.26 ± 0.76 ab |
| N, % a.d.m. | 4.35 ± 0.22 a | 4.51 ± 0.23 a | 4.33 ± 0.22 a | 4.32 ± 0.22 a | 5.25 ± 0.26 a | 4.92 ± 0.25 a | 5.13 ± 0.26 a | 5.17 ± 0.26 a | 5.21 ± 0.26 a | 5.48 ± 0.27 a | 5.48 ± 0.27 a | 5.34 ± 0.27 a | 4.11 ± 0.21 b | 4.80 ± 0.24 a | 4.37 ± 0.22 ab | 4.50 ± 0.23 ab |
| P, % a.d.m. | 0.81 ± 0.04 a | 0.81 ± 0.04 a | 0.76 ± 0.04 a | 0.79 ± 0.04 a | 0.68 ± 0.03 ab | 0.74 ± 0.04 a | 0.66 ± 0.03 b | 0.51 ± 0.03 c | 0.65 ± 0.03 b | 0.72 ± 0.04 a | 0.68 ± 0.03 ab | 0.65 ± 0.03 b | 0.68 ± 0.03 a | 0.73 ± 0.04 a | 0.69 ± 0.03 a | 0.71 ± 0.04 a |
| K, % a.d.m. | 8.80 ± 0.44 b | 9.75 ± 0.49 a | 8.70 ± 0.44 b | 7.75 ± 0.39 c | 4.29 ± 0.21 c | 5.20 ± 0.26 a | 4.53 ± 0.23 bc | 4.79 ± 0.24 ab | 2.97 ± 0.15 b | 3.32 ± 0.17 a | 3.21 ± 0.16 ab | 2.95 ± 0.15 b | 2.82 ± 0.14 b | 3.46 ± 0.17 a | 2.90 ± 0.15 b | 2.96 ± 0.15 b |
| Ca, % a.d.m. | 1.52 ± 0.08 a | 1.49 ± 0.07 a | 1.46 ± 0.07 a | 1.50 ± 0.08 a | 5.88 ± 0.29 a | 5.74 ± 0.29 a | 5.99 ± 0.30 a | 5.88 ± 0.29 a | 3.26 ± 0.16 b | 3.26 ± 0.16 b | 3.41 ± 0.17 ab | 3.68 ± 0.18 a | 3.10 ± 0.16 a | 3.13 ± 0.16 a | 3.38 ± 0.17 a | 3.25 ± 0.16 a |
| Mg, % a.d.m. | 0.43 ± 0.02 c | 0.45 ± 0.02 bc | 0.48 ± 0.02 ab | 0.52 ± 0.03 a | 0.44 ± 0.02 ab | 0.43 ± 0.02 ab | 0.46 ± 0.02 a | 0.41 ± 0.02 b | 0.56 ± 0.03 b | 0.60 ± 0.03 b | 0.67 ± 0.03 a | 0.69 ± 0.03 a | 0.43 ± 0.02 b | 0.54 ± 0.03 a | 0.50 ± 0.02 b | 0.49 ± 0.02 b |
| Fe, mg/kg a.d.m. | 75.0 ± 3.8 a | 78.6 ± 3.9 ab | 80.5 ± 4.0 ab | 85.6 ± 4.3 a | 78.5 ± 3.9 a | 63.5 ± 3.2 b | 65.9 ± 3.3 b | 56.2 ± 2.8 c | 70.5 ± 3.5 a | 65.6 ± 3.3 ab | 62.2 ± 3.1 b | 60.8 ± 3.0 b | 59.8 ± 3.0 b | 72.4 ± 3.6 a | 67.4 ± 3.4 a | 67.2 ± 3.4 a |
| Mn, mg/kg a.d.m. | 60.2 ± 3.0 b | 67.3 ± 3.4 a | 70.4 ± 3.5 a | 65.1 ± 3.3 ab | 135.6 ± 6.8 bc | 124.4 ± 6.2 c | 146.6 ± 7.3 ab | 152.8 ± 7.6 a | 239.2 ± 12.0 b | 251.8 ± 12.6 b | 288.5 ± 14.4 a | 301.8 ± 15.1 a | 98.0 ± 4.9 b | 115.8 ± 5.8 a | 114.0 ± 5.7 a | 118.7 ± 5.9 a |
| Cu, mg/kg a.d.m. | 5.03 ± 0.25 a | 4.86 ± 0.24 a | 4.90 ± 0.25 a | 4.78 ± 0.24 a | 4.47 ± 0.22 ab | 4.70 ± 0.24 a | 4.18 ± 0.21 b | 2.61 ± 0.13 c | 8.16 ± 0.41 ab | 7.30 ± 0.37 c | 7.87 ± 0.39 bc | 8.92 ± 0.45 a | 4.60 ± 0.23 a | 4.83 ± 0.24 a | 4.71 ± 0.24 a | 4.64 ± 0.23 a |
| Zn, mg/kg a.d.m. | 47.4 ± 2.4 a | 47.5 ± 2.4 a | 44.1 ± 2.2 a | 44.8 ± 2.2 a | 68.1 ± 3.4 a | 61.4 ± 3.1 b | 56.1 ± 2.8 b | 58.3 ± 2.9 b | 63.5 ± 3.2 a | 68.5 ± 3.4 a | 66.4 ± 3.3 a | 67.3 ± 3.4 a | 18.2 ± 0.9 b | 22.1 ± 1.1 a | 19.7 ± 1.0 b | 23.7 ± 1.2 a |
| Root-Inhabited Environment * | Plant | ||||||
|---|---|---|---|---|---|---|---|
| Height, cm | Rosette Diameter, cm | Number of Leaves, pcs. | Raw Mass, g | Dry Mass, g | % Dry Matter | Leaves Square, cm2 | |
| Leaf salad cv. Typhoon | |||||||
| TAS (control) | 14.0 ± 1.2 b | 23.0 ± 1.4 b | 13.8 ± 1.3 a | 46.6 ± 7.0 b | 2.40 ± 0.59 b | 5.3 ± 0.3 ab | 1593.7 ± 378 a |
| TAS + SC (standard) | 15.8 ± 1.2 ab | 25.4 ± 1.9 ab | 14.0 ± 1.5 a | 50.6 ± 8.2 ab | 2.42 ± 0.76 ab | 4.8 ± 0.2 b | 1728.6 ± 518 a |
| TAS + GG | 16.1 ± 1.2 a | 23.9 ± 1.4 ab | 13.0 ± 1.3 a | 49.2 ± 9.9 ab | 2.50 ± 0.56 ab | 5.1 ± 0.3 b | 1840.4 ± 374 a |
| TAS + mGG | 15.9 ± 0.9 ab | 26.2 ± 1.9 a | 15.0 ± 1.0 a | 61.5 ± 1.1 a | 3.53 ± 0.39 a | 5.7 ± 0.3 a | 2215.9 ± 245 a |
| Radish cv. Sacharok | |||||||
| TAS (control) | 24.4 ± 0.9 a | 23.1 ± 0.9 ab | 5.8 ± 0.6 a | 22.2 ± 3.4 a | 1.64 ± 0.26 ab | 7.4 ± 0.2 b | 828.7 ± 132 a |
| TAS + SC (standard) | 23.5 ± 1.1 a | 24.7 ± 1.5 a | 5.7 ± 0.4 a | 23.7 ± 5.0 a | 1.66 ± 0.35 ab | 7.0 ± 0.3 b | 949.8 ± 200 a |
| TAS + GG | 22.9 ± 1.8 a | 22.2 ± 1.4 ab | 5.9 ± 0.2 a | 22.4 ± 3.3 a | 1.78 ± 0.26 a | 7.9 ± 0.3 a | 880.1 ± 149 a |
| TAS + mGG | 22.6 ± 1.0 a | 21.9 ± 1.1 b | 5.5 ± 0.3 a | 19.8 ± 2.4 a | 1.27 ± 0.15 b | 6.4 ± 0.2 c | 741.7 ± 96 a |
| Root-Inhabited Environment * | Height, cm | Leaves/Plant | Stems/Plant | Roots/Plant | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Leaves, pcs | Leaves Square, cm2 | Raw Mass, g | Dry Mass, g | % Dry Matter | Cross-Sectional Area, cm2 | Raw Mass, g | Dry Mass, g | % Dry Matter | Height, cm | Raw Mass, g | Dry Mass, g | % Dry Matter | ||
| Cucumber Hybrid F1 Neva | ||||||||||||||
| TAS (control) | 9.5 ± 1.5 c | 3.0 ± 0.0 c | 607 ± 74 d | 10.8 ± 1.3 c | 1.64 ± 0.07 c | 15.1 ± 0.3 a | 33.0 ± 0.9 d | 6.0 ± 0.7 c | 0.31 ± 0.04 c | 5.2 ± 0.2 a | 33.3 ± 0.9 a | 7.9 ± 1.5 c | 0.25 ± 0.05 c | 3.2 ± 0.2 c |
| TAS + SC (standard) | 12.2 ± 1.2 b | 3.6 ± 0.4 bc | 925 ± 98 c | 15.3 ± 3.9 bc | 2.24 ± 0.58 bc | 14.6 ± 0.2 a | 48.1 ± 2.7 c | 8.5 ± 2.7 bc | 0.44 ± 0.13 bc | 5.1 ± 0.1 a | 37.0 ± 5.3 a | 10.7 ± 3.0 bc | 0.52 ± 0.03 bc | 4.9 ± 0.3 ab |
| TAS + GG | 14.2 ± 1.6 ab | 4.0 ± 0.2 b | 1193 ± 139 b | 19.8 ± 2.2 ab | 2.90 ± 0.57 ab | 14.7 ± 0.3 a | 65.0 ± 0.8 b | 11.5 ± 1.9 ab | 0.60 ± 0.15 ab | 5.2 ± 0.2 a | 35.0 ± 2.7 a | 14.0 ± 2.7 ab | 0.66 ± 0.21 ab* | 4.7 ± 0.3 b |
| TAS + mGG | 15.3 ± 1.1 a | 4.6 ± 0.3 a | 1469 ± 193 a | 22.7 ± 2.7 a | 3.38 ± 0.41 a | 14.9 ± 0.2 a | 73.6 ± 3.4 a | 13.9 ± 2.3 a | 0.73 ± 0.11 a | 5.3 ± 0.2 a | 35.0 ± 2.4 a | 17.3 ± 3.4 a | 0.91 ± 0.11 a* | 5.3 ± 0.2 a |
| Tomato cv. Natasha | ||||||||||||||
| TAS (control) | 16.0 ± 0.9 a | 8.4 ± 0.4 a | 818 ± 73 b | 25.0 ± 2.2 c | 2.7 ± 0.2 b | 10.6 ± 0.4 b | 30.6 ± 1.8 c | 9.6 ± 1.5 b | 1.0 ± 0.2 b | 10.2 ± 0.2 b | 30.8 ± 1.7 a | 12.7 ± 0.9 b | 0.8 ± 0.1 c | 6.5 ± 0.2 b |
| TAS + SC (standard) | 16.8 ± 0.8 a | 8.4 ± 0.4 a | 1141 ± 95 a | 30.5 ± 2.5 b | 2.7 ± 0.2 b | 8.7 ± 0.5 c | 29.8 ± 1.7 c | 9.8 ± 0.6 b | 0.9 ± 0.1 b | 8.7 ± 0.4 c | 34.2 ± 3.2 a | 13.3 ± 1.8 ab | 1.0 ± 0.1 bc | 7.6 ± 0.3 a |
| TAS + GG | 16.8 ± 0.7 a | 8.4 ± 0.4 a | 1214 ± 131 a | 35.6 ± 3.9 a | 4.4 ± 0.5 a | 12.3 ± 0.4 a | 34.8 ± 2.1 b | 12.9 ± 1.0 a | 1.4 ± 0.2 a | 11.1 ± 0.3 a | 35.8 ± 4.6 a | 16.5 ± 1.4 a | 1.4 ± 0.2 a | 8.2 ± 0.3 a |
| TAS + mGG | 17.4 ± 1.1 a | 8.6 ± 0.4 a | 1276 ± 128 a | 39.0 ± 3.9 a | 4.4 ± 0.4 a | 11.2 ± 0.2 b | 39.4 ± 2.2 a | 14.7 ± 1.6 a | 1.5 ± 0.2 a | 10.4 ± 0.2 b | 30.6 ± 1.6 a | 15.9 ± 2.5 ab | 1.2 ± 0.2 ab | 7.8 ± 0.4 a |
| Indicators | Radish cv. Sacharok | Cucumber Hybrid F1 Neva | Tomato cv. Natasha | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root-Inhabited Environment * | ||||||||||||
| TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 19 | 10 | 11 | 12 | 13 |
| Raw ash, % a.d.m. | 16.24 ± 0.81 a | 15.85 ± 0.79 a | 16.14 ± 0.81 a | 15.82 ± 0.79 a | 11.83 ± 0.59 a | 12.15 ± 0.61 a | 12.10 ± 0.61 a | 12.22 ± 0.61 a | 9.84 ± 0.49 b | 9.93 ± 0.50 ab | 10.86 ± 0.54 a | 9.89 ± 0.49 ab |
| N, % a.d.m. | 2.83 ± 0.14 a | 3.06 ± 0.15 a | 2.97 ± 0.15 a | 2.97 ± 0.15 a | 3.87 ± 0.19 b | 3.85 ± 0.19 b | 4.19 ± 0.21 ab | 4.32 ± 0.22 a | 2.60 ± 0.13 a | 2.81 ± 0.14 a | 2.67 ± 0.13 a | 2.80 ± 0.14 a |
| P, % a.d.m. | 0.76 ± 0.04 a | 0.79 ± 0.04 a | 0.73 ± 0.04 a | 0.76 ± 0.04 a | 0.94 ± 0.05 b | 0.97 ± 0.05 b | 1.06 ± 0.05 a | 0.99 ± 0.05 b | 0.61 ± 0.03 c | 0.72 ± 0.03 a | 0.60 ± 0.04 bc | 0.68 ± 0.03 ab |
| K, % a.d.m. | 7.42 ± 0.37 a | 7.25 ± 0.36 a | 7.41 ± 0.37 a | 7.41 ± 0.37 a | 5.45 ± 0.27 a | 5.43 ± 0.27 a | 5.68 ± 0.28 a | 5.82 ± 0.29 a | 4.16 ± 0.21 b | 5.02 ± 0.25 a | 4.34 ± 0.22 b | 4.18 ± 0.21 b |
| Ca, % a.d.m. | 0.60 ± 0.03 a | 0.60 ± 0.03 a | 0.60 ± 0.03 a | 0.58 ± 0.03 a | 0.52 ± 0.03 b | 0.52 ± 0.03 b | 0.55 ± 0.03 b | 0.70 ± 0.04 a | 0.18 ± 0.01 a | 0.19 ± 0.01 a | 0.18 ± 0.01 a | 0.18 ± 0.01 a |
| Mg, % a.d.m. | 0.17 ± 0.01 a | 0.17 ± 0.01 a | 0.18 ± 0.01 a | 0.18 ± 0.01 a | 0.26 ± 0.01 a | 0.26 ± 0.01 a | 0.26 ± 0.01 a | 0.28 ± 0.01 a | 0.14 ± 0.01 b | 0.16 ± 0.01 a | 0.17 ± 0.01 a | 0.17 ± 0.01 a |
| Fe, mg/kg a.d.m. | 17.9 ± 0.9 d | 28.2 ± 1.4 a | 20.5 ± 1.0 c | 23.1 ± 1.2 b | 31.8 ± 1.6 c | 35.2 ± 1.8 c | 43.5 ± 2.2 b | 55.2 ± 2.8 a | 30.3 ± 1.5 b | 37.0 ± 1.8 a | 35.5 ± 1.8 a | 36.6 ± 1.8 a |
| Mn, mg/kg a.d.m. | 17.9 ± 0.9 a | 17.0 ± 0.8 a | 16.4 ± 0.8 a | 17.3 ± 0.9 a | 36.4 ± 1.8 a | 36.0 ± 1.8 a | 37.9 ± 1.9 a | 38.4 ± 1.9 a | 11.8 ± 0.6 b | 13.9 ± 0.7 a | 14.3 ± 0.7 a | 14.9 ± 0.7 a |
| Cu, mg/kg a.d.m. | 2.59 ± 0.13 a | 2.54 ± 0.13 a | 2.55 ± 0.13 a | 2.57 ± 0.13 a | 10.30 ± 0.52 c | 9.90 ± 0.50 c | 15.10 ± 0.76 b | 19.20 ± 0.96 a | 10.02 ± 0.50 c | 12.80 ± 0.64 a | 11.50 ± 0.58 b | 11.70 ± 0.59 b |
| Zn, mg/kg a.d.m. | 47.8 ± 2.4 ab | 48.0 ± 2.4 ab | 44.1 ± 2.2 b | 49.0 ± 2.4 a | 28.3 ± 1.4 c | 27.2 ± 1.4 c | 44.5 ± 2.2 b | 51.2 ± 2.6 a | 16.2 ± 0.8 c | 18.3 ± 0.9 b | 17.6 ± 0.9 bc | 21.4 ± 1.1 a |
| Indicators | Leaf Salad cv. Typhoon | Radish cv. Sacharok | Cucumber Hybrid F1 Neva | Tomato cv. Natasha | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root-Inhabited Environment * | ||||||||||||||||
| TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | TAS (Control) | TAS + SC (Standard) | TAS + GG | TAS + mGG | |
| Humidity,% | 94.56 ± 4.73 a | 93.44 ± 4.67 a | 95.10 ± 4.76 a | 94.87 ± 4.74 a | 94.80 ± 4.74 a | 94.40 ± 4.72 a | 94.40 ± 4.72 a | 94.60 ± 4.73 a | 96.80 ± 4.84 a | 96.60 ± 4.83 a | 96.80 ± 4.84 a | 96.80 ± 4.84 a | 94.89 ± 4.74 a | 94.84 ± 4.74 a | 94.82 ± 4.74 a | 94.64 ± 4.73 a |
| % dry matter | 5.44 ± 0.27 b | 6.56 ± 0.33 a | 4.90 ± 0.25 b | 5.13 ± 0.26 b | 5.20 ± 0.26 a | 5.60 ± 0.28 a | 5.60 ± 0.28 a | 5.40 ± 0.27 a | 3.20 ± 0.16 a | 3.40 ± 0.17 a | 3.20 ± 0.16 a | 3.40 ± 0.17 a | 5.11 ± 0.26 a | 5.16 ± 0.26 a | 5.18 ± 0.26 a | 5.36 ± 0.27 a |
| Vitamin C, mg/100 g FM | 15.84 ± 0.79 b | 12.10 ± 0.61 c | 17.16 ± 0.86 ab | 18.25 ± 0.91 a | 21.30 ± 1.07 a | 18.70 ± 0.94 b | 22.00 ± 1.10 a | 22.00 ± 1.12 a | 4.62 ± 0.23 b | 5.06 ± 0.25 b | 9.02 ± 0.45 a | 9.41 ± 0.47 a | 15.20 ± 0.76 b | 16.50 ± 0.83 b | 19.80 ± 0.99 a | 20.90 ± 1.05 a |
| Nitrates, mg/kg FM | 1200.4 ± 60.02 b | 1807.0 ± 90.35 a | 664.9 ± 33.25 c | 715.2 ± 35.76 c | 1192.0 ± 71.0 a | 1186.0 ± 76.6 a | 1206.0 ± 62.4 a | 1171.0 ± 58.6 a | 277.0 ± 13.85 b | 340.0 ± 17.00 a | 283.0 ± 14.15 b | 297.0 ± 14.85 b | 150.0 ± 7.50 a | 105.0 ± 5.25 b | 71.3 ± 3.57 c | 66.6 ± 3.33 c |
| Plant | Growing Equipment | Method of Plant Cultivation | Nutrient Solution | Method of Treatment with Test Substance | Condition | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Air Temperature: Day/Night | Air Humidity | Light Source/Duration of the Light Period | Light Intensity | pH of Root Inhabitat Zone, Relative Units | EC, mS cm−1 | |||||
| Leaf salad | Plant growing light equipment for plants with High up to 50 cm | Panoponic [25] | Knop | Introduction the seeds | +20–+22 °C/ +16–+18 °C | 60–70% | High-pressure sodium lamps (DNaZ-400, “Reflax” LLC, Moscow, Russia)/14 h per day | 80–90 W/m2 in the PAR | 5.8–6.0 | 1.5 |
| Radish | +20–+22 °C/ +16–+18 °C | 60–70% | ||||||||
| Tomato | +22–+24 °C/ +18–+20 °C | 60–70% | High-pressure sodium lamps (DNaZ-400, “Reflax” LLC, Moscow, Russia)/16 h per day | |||||||
| Cucum-ber | Plant growing light equipment for plants with High up to 200 cm | modified Knop [38] | +22–+24 °C/ +18–+20 °C | 75–80% | High-pressure sodium lamps (DNaZ-400, “Reflax” LLC, Moscow, Russia)/14 h per day | Nutrint solution 6.0–6.2 | Nutrint solution 1.6 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panova, G.G.; Krasnopeeva, E.L.; Laishevkina, S.G.; Kuleshova, T.E.; Udalova, O.R.; Khomyakov, Y.V.; Mirskaya, G.V.; Vertebny, V.E.; Zhuravleva, A.S.; Shevchenko, N.N.; et al. Polymer Gel Substrate: Synthesis and Application in the Intensive Light Artificial Culture of Agricultural Plants. Gels 2023, 9, 937. https://doi.org/10.3390/gels9120937
Panova GG, Krasnopeeva EL, Laishevkina SG, Kuleshova TE, Udalova OR, Khomyakov YV, Mirskaya GV, Vertebny VE, Zhuravleva AS, Shevchenko NN, et al. Polymer Gel Substrate: Synthesis and Application in the Intensive Light Artificial Culture of Agricultural Plants. Gels. 2023; 9(12):937. https://doi.org/10.3390/gels9120937
Chicago/Turabian StylePanova, Gayane G., Elena L. Krasnopeeva, Svetlana G. Laishevkina, Tatiana E. Kuleshova, Olga R. Udalova, Yuriy V. Khomyakov, Galina V. Mirskaya, Vitaly E. Vertebny, Anna S. Zhuravleva, Natalia N. Shevchenko, and et al. 2023. "Polymer Gel Substrate: Synthesis and Application in the Intensive Light Artificial Culture of Agricultural Plants" Gels 9, no. 12: 937. https://doi.org/10.3390/gels9120937
APA StylePanova, G. G., Krasnopeeva, E. L., Laishevkina, S. G., Kuleshova, T. E., Udalova, O. R., Khomyakov, Y. V., Mirskaya, G. V., Vertebny, V. E., Zhuravleva, A. S., Shevchenko, N. N., & Yakimansky, A. V. (2023). Polymer Gel Substrate: Synthesis and Application in the Intensive Light Artificial Culture of Agricultural Plants. Gels, 9(12), 937. https://doi.org/10.3390/gels9120937